

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50502344 (Talinolol) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University of Frankfurt Curated by ChEMBL | Assay Description Binding affinity to beta1 adrenergic receptor (unknown origin) by radioligand binding assay | ACS Med Chem Lett 10: 899-903 (2019) Article DOI: 10.1021/acsmedchemlett.9b00075 BindingDB Entry DOI: 10.7270/Q2R214M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50502344 (Talinolol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University of Frankfurt Curated by ChEMBL | Assay Description Binding affinity to beta2 adrenergic receptor (unknown origin) by radioligand binding assay | ACS Med Chem Lett 10: 899-903 (2019) Article DOI: 10.1021/acsmedchemlett.9b00075 BindingDB Entry DOI: 10.7270/Q2R214M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50312882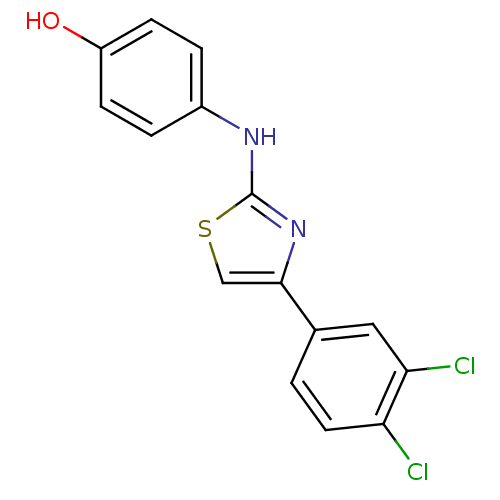 (4-(4-(3,4-dichlorophenyl)thiazol-2-ylamino)phenol ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM91660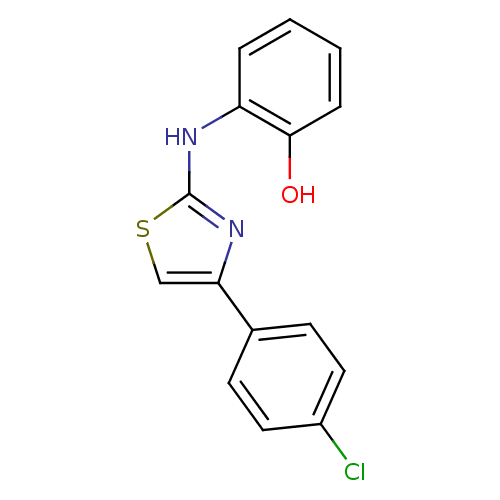 (N-aryl-4-aryl-1,3-thiazole-2-amine, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50444531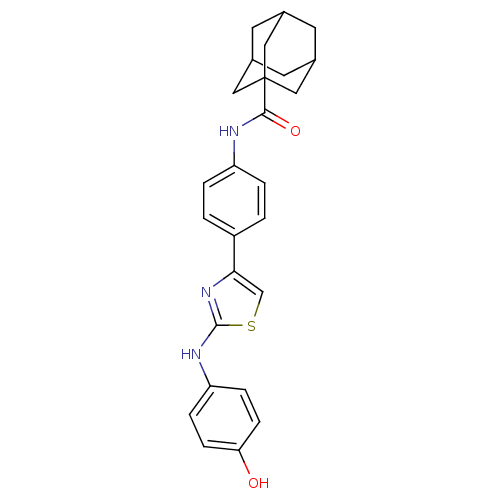 (CHEMBL3099680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant 5-lipoxygenase (unknown origin) expressed in Escherichia coli BL21(DE3) using arachidonic acid as substrate incubated for 1... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051932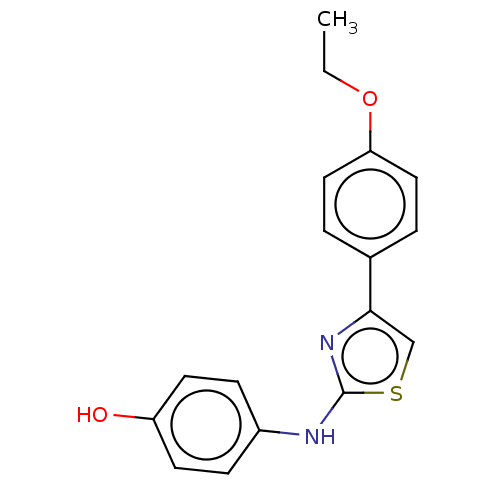 (CHEMBL3318370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50312869 (4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051857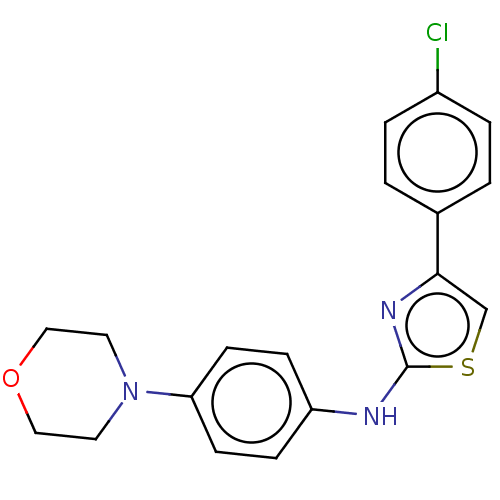 (CHEMBL3318382) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50382192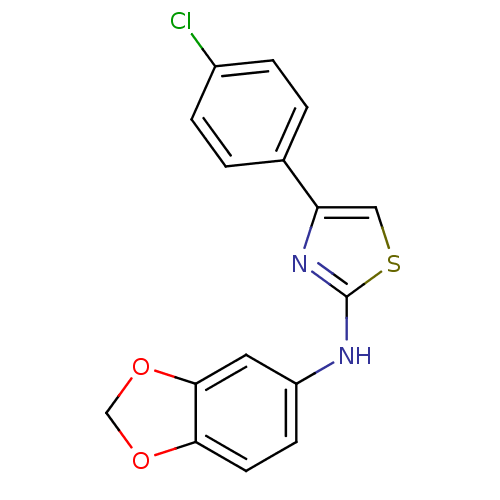 (CHEMBL2023847) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM91656 (N-aryl-4-aryl-1,3-thiazole-2-amine, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051863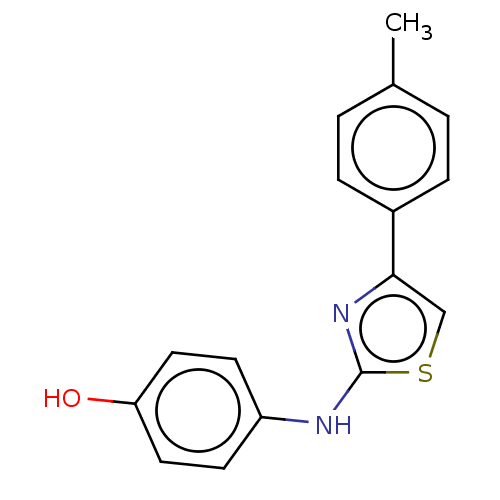 (CHEMBL1081479) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50502345 (CHEMBL4566250) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University of Frankfurt Curated by ChEMBL | Assay Description Inhibition of recombinant human full length soluble epoxide hydrolase (1 to 555 residues) expressed in Escherichia coli BL21(DE3) using non-fluoresce... | ACS Med Chem Lett 10: 899-903 (2019) Article DOI: 10.1021/acsmedchemlett.9b00075 BindingDB Entry DOI: 10.7270/Q2R214M7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50293576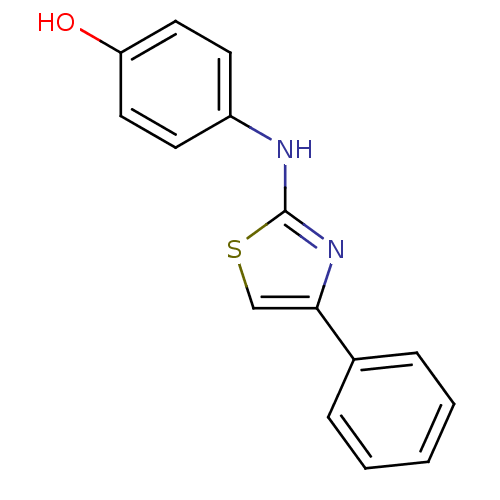 (4-(4-phenylthiazol-2-ylamino)phenol | 4131JH0380 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM25737 (12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe University Curated by ChEMBL | Assay Description Inhibition of sEH using (3-Phenyl-oxiranyl)-acetic acid cyano-(6-methoxy-naphthalen-2-yl)-methyl ester) as substrate incubated for 15 mins prior to s... | Bioorg Med Chem Lett 22: 6762-5 (2012) Article DOI: 10.1016/j.bmcl.2012.08.066 BindingDB Entry DOI: 10.7270/Q2028SNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50444531 (CHEMBL3099680) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50293576 (4-(4-phenylthiazol-2-ylamino)phenol | 4131JH0380 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human PMNL using arachidonic acid as substrate preincubated for 15 mins before substrate addition measured after 10 m... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051856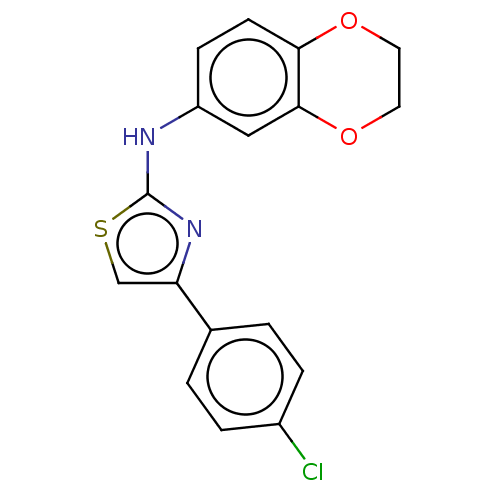 (CHEMBL3318381) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051937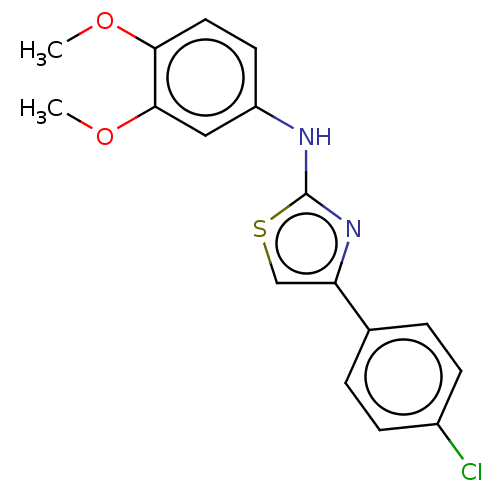 (CHEMBL3318373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM49887 (4-(4-methoxyphenyl)-N-phenyl-1,3-thiazol-2-amine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051855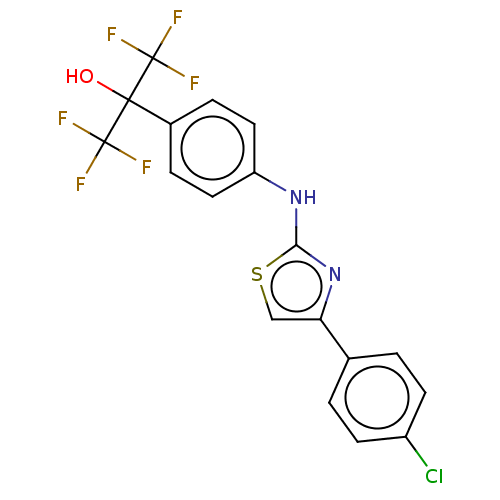 (CHEMBL3318380) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051854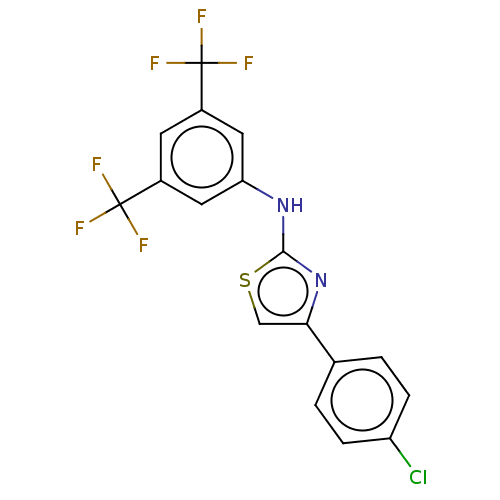 (CHEMBL3318379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051934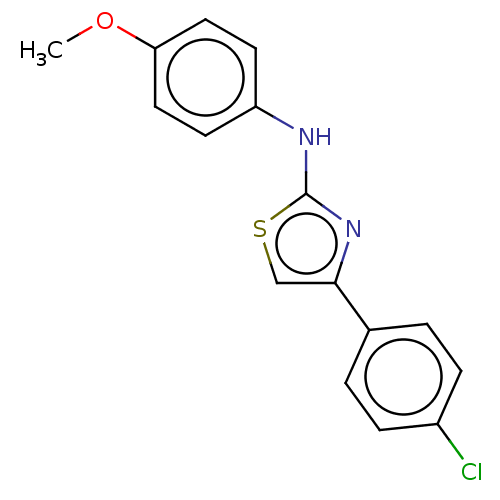 (CHEMBL1080391) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051862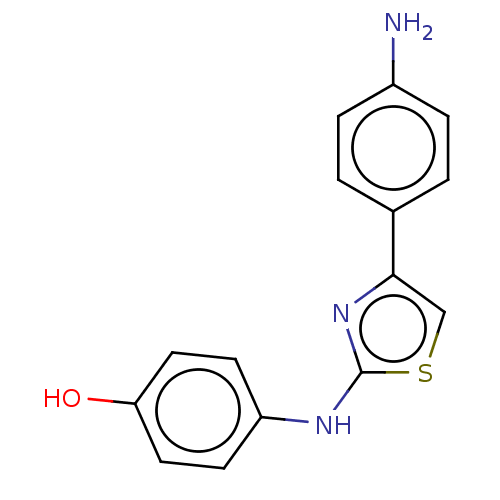 (CHEMBL3318369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50384785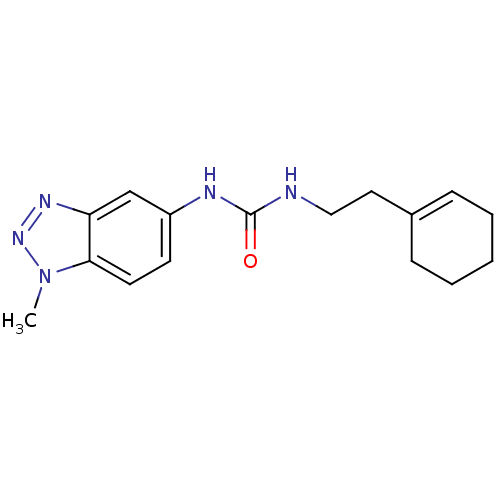 (CHEMBL2037376) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of soluble epoxide hydrolase using (3-Phenyl-oxiranyl)-acetic acid cyano-(6-methoxy-naphthalen-2-yl)-methyl ester) as substrate incubated ... | ACS Med Chem Lett 3: 155-158 (2012) Article DOI: 10.1021/ml200286e BindingDB Entry DOI: 10.7270/Q2J10468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051858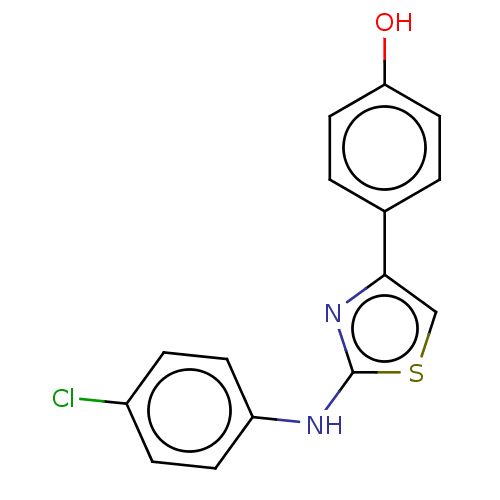 (CHEMBL3318383) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051853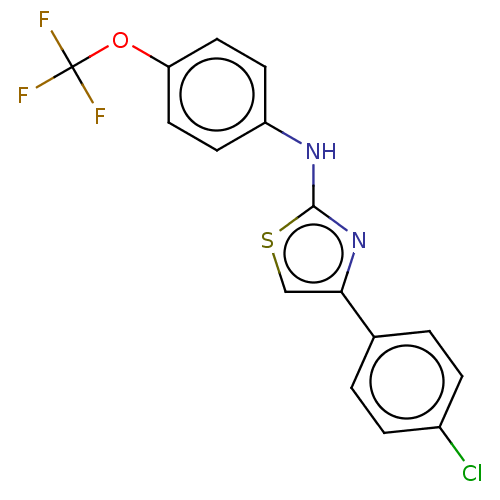 (CHEMBL3318378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051859 (CHEMBL3318384) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051852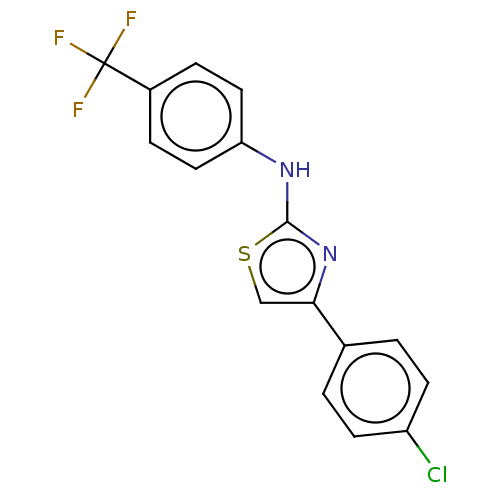 (CHEMBL3318377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051938 (CHEMBL3318374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051932 (CHEMBL3318370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human PMNL using arachidonic acid as substrate preincubated for 15 mins before substrate addition measured after 10 m... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051936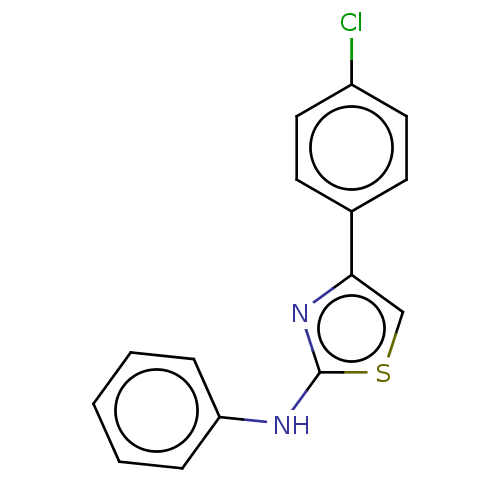 (CHEMBL68504) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM91656 (N-aryl-4-aryl-1,3-thiazole-2-amine, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human PMNL using arachidonic acid as substrate preincubated for 15 mins before substrate addition measured after 10 m... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50312869 (4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human PMNL using arachidonic acid as substrate preincubated for 15 mins before substrate addition measured after 10 m... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051851 (CHEMBL3318376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM91660 (N-aryl-4-aryl-1,3-thiazole-2-amine, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human PMNL using arachidonic acid as substrate preincubated for 15 mins before substrate addition measured after 10 m... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051863 (CHEMBL1081479) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human PMNL using arachidonic acid as substrate preincubated for 15 mins before substrate addition measured after 10 m... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50312882 (4-(4-(3,4-dichlorophenyl)thiazol-2-ylamino)phenol ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human PMNL using arachidonic acid as substrate preincubated for 15 mins before substrate addition measured after 10 m... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM49484 ((4-methoxyphenyl)-(4-phenylthiazol-2-yl)amine | ML...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051933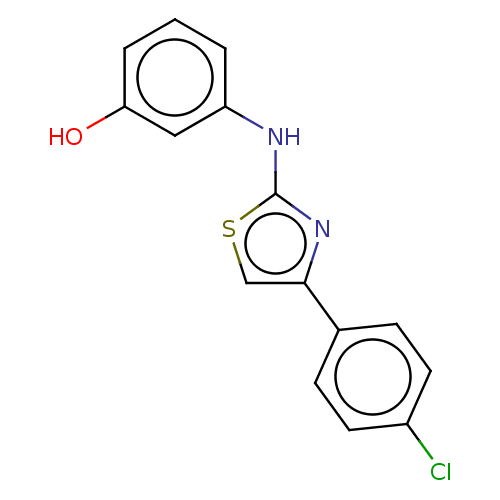 (CHEMBL1087624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM49887 (4-(4-methoxyphenyl)-N-phenyl-1,3-thiazol-2-amine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human PMNL using arachidonic acid as substrate preincubated for 15 mins before substrate addition measured after 10 m... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50384786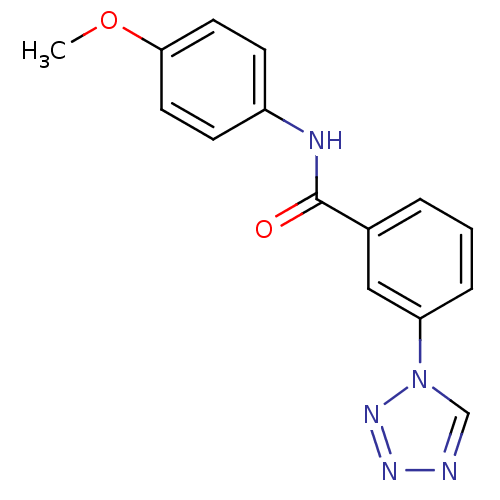 (CHEMBL1364973) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of soluble epoxide hydrolase using (3-Phenyl-oxiranyl)-acetic acid cyano-(6-methoxy-naphthalen-2-yl)-methyl ester) as substrate incubated ... | ACS Med Chem Lett 3: 155-158 (2012) Article DOI: 10.1021/ml200286e BindingDB Entry DOI: 10.7270/Q2J10468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50384782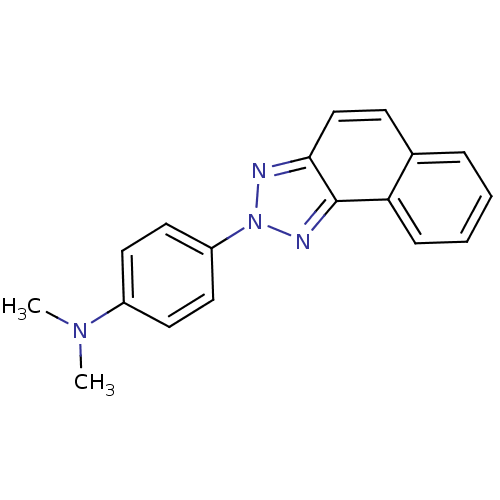 (CHEMBL2037373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant 5-lipooxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated for 15 mins prior to subst... | ACS Med Chem Lett 3: 155-158 (2012) Article DOI: 10.1021/ml200286e BindingDB Entry DOI: 10.7270/Q2J10468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50384783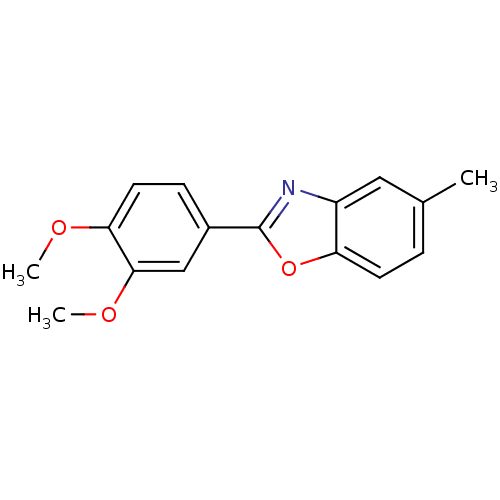 (CHEMBL2037374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant 5-lipooxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated for 15 mins prior to subst... | ACS Med Chem Lett 3: 155-158 (2012) Article DOI: 10.1021/ml200286e BindingDB Entry DOI: 10.7270/Q2J10468 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051935 (CHEMBL3318372) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50502344 (Talinolol) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University of Frankfurt Curated by ChEMBL | Assay Description Inhibition of recombinant human full length soluble epoxide hydrolase (1 to 555 residues) expressed in Escherichia coli BL21(DE3) using non-fluoresce... | ACS Med Chem Lett 10: 899-903 (2019) Article DOI: 10.1021/acsmedchemlett.9b00075 BindingDB Entry DOI: 10.7270/Q2R214M7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051861 (CHEMBL3318386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of partially purified recombinant 5-lipoxygenase (unknown origin) using arachidonic acid as substrate preincubated for 15 mins before subs... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051853 (CHEMBL3318378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human PMNL using arachidonic acid as substrate preincubated for 15 mins before substrate addition measured after 10 m... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50444538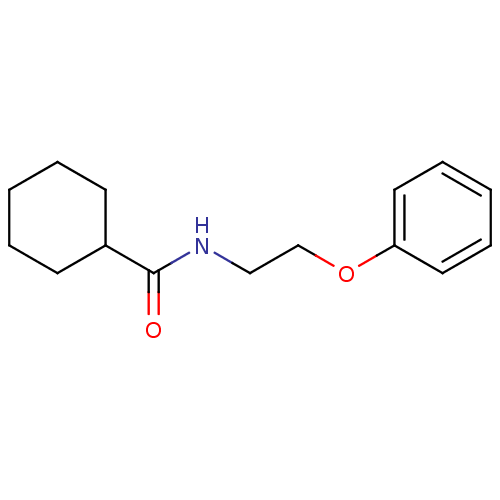 (CHEMBL1313977) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Curated by ChEMBL | Assay Description Inhibition of recombinant soluble epoxide hydrolase (unknown origin) using PHOME as substrate incubated 15 mins prior to substrate addition measured ... | ACS Med Chem Lett 4: 1169-72 (2013) Article DOI: 10.1021/ml4002562 BindingDB Entry DOI: 10.7270/Q228092M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051856 (CHEMBL3318381) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human PMNL using arachidonic acid as substrate preincubated for 15 mins before substrate addition measured after 10 m... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50051858 (CHEMBL3318383) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human PMNL using arachidonic acid as substrate preincubated for 15 mins before substrate addition measured after 10 m... | Eur J Med Chem 84: 302-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.025 BindingDB Entry DOI: 10.7270/Q2WM1G2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 118 total ) | Next | Last >> |