

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194146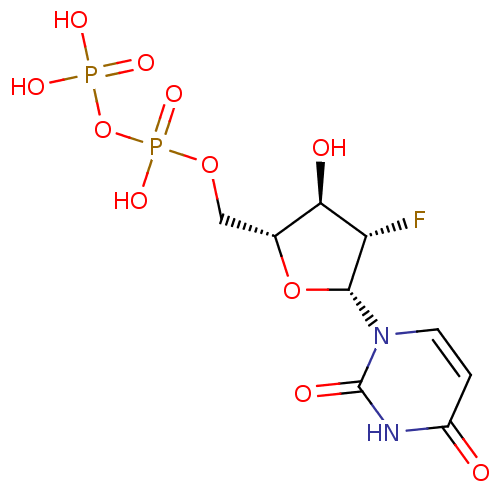 ((2R,3S,4R,5R)-1-(5-(diphosphoryloxymethyl)-3-fluor...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194161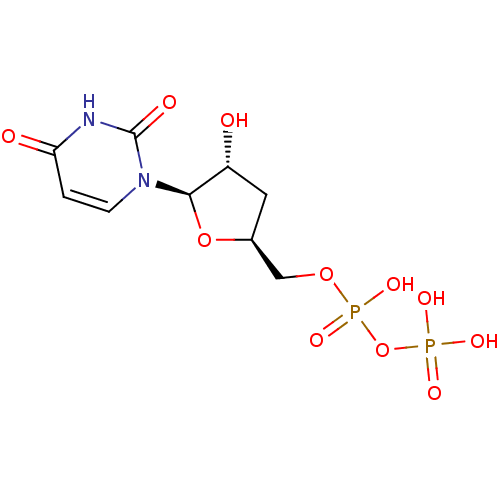 ((2R,3R,5S)-1-(5-(diphosphoryloxymethyl)-3-hydroxyt...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194147 (4-Thio-UDP | CHEMBL384992 | [(2R,3S,4R,5R)-3,4-dih...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194148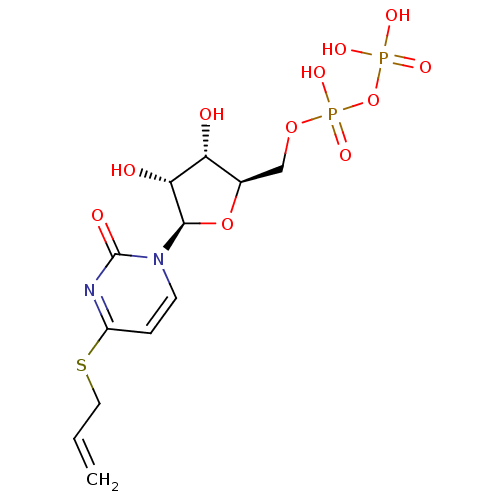 ((2R,3R,4S,5R)-4-(allylthio)-1-(3,4-dihydroxy-5-(di...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 560 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM92459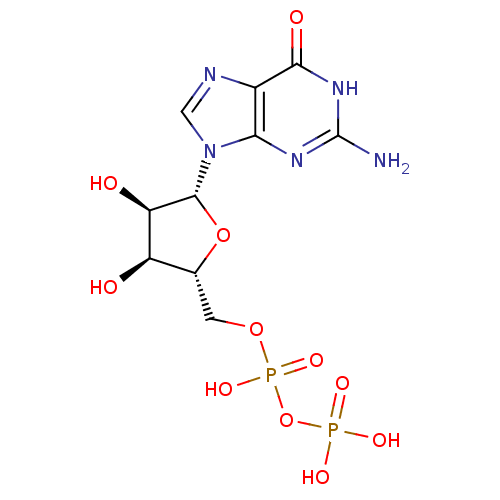 (CHEMBL384759 | GDP | Guanosine Diphosphate) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50368125 (ADENOSINE DIPHOSPHATE | ADP) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194150 ((1S,3R,4R,5S)-1-(4-hydroxy-1-(diphosphoryloxymethy...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194152 ((2R,3R,4S,5R)-1-(3,4-dihydroxy-5-(diphosphoryloxym...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194153 (5'-CDP | CDP | CHEMBL425252 | Cytidine | Cytidine ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.80E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194151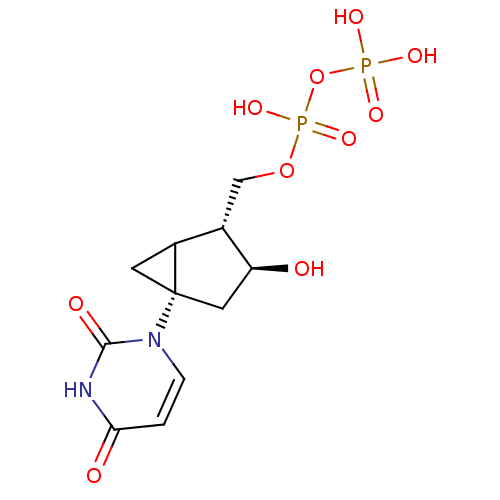 (CHEMBL212090 | [(2R,3S,5S)-5-(2,4-dioxo-3,4-dihydr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194154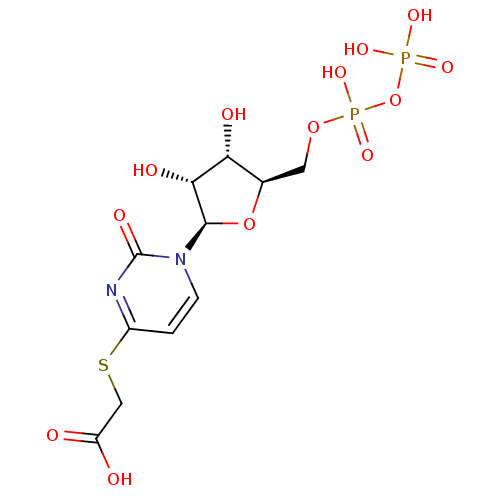 ((2R,3R,4S,5R)-2-(1-(3,4-dihydroxy-5-(diphosphorylo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194155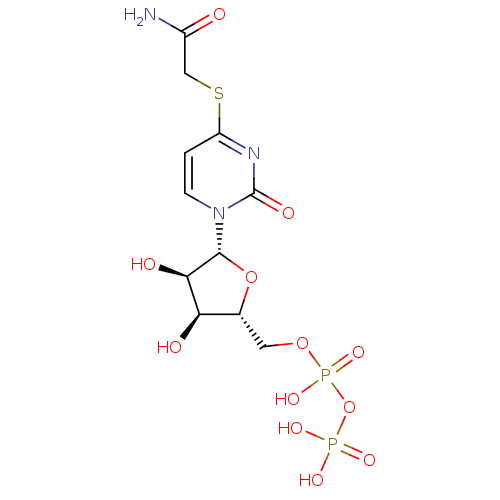 ((2R,3R,4S,5R)-2-(1-(3,4-dihydroxy-5-(diphosphorylo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50179185 (((2R,3S,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194156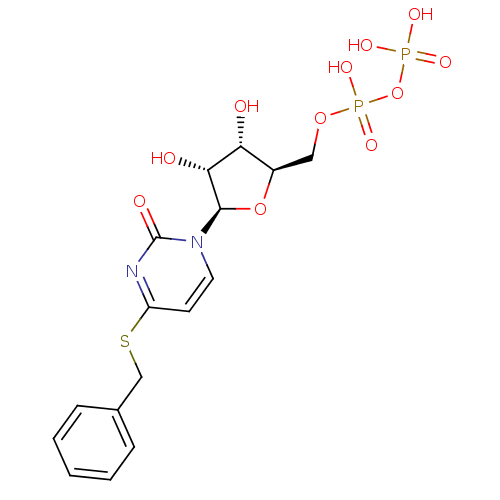 ((2R,3R,4S,5R)-4-(benzylthio)-1-(3,4-dihydroxy-5-(d...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194157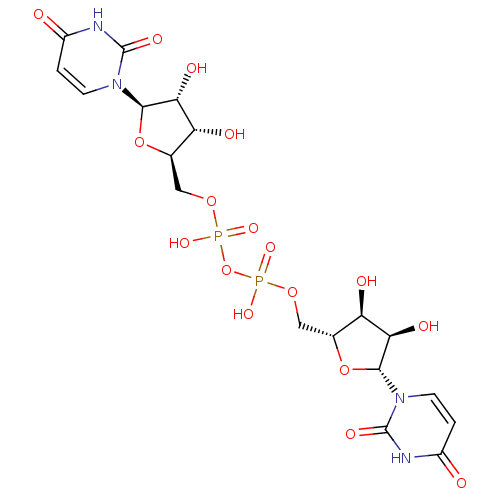 (CHEMBL378445 | {[(2R,3S,4R,5R)-5-(2,4-dioxo-1,2,3,...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194158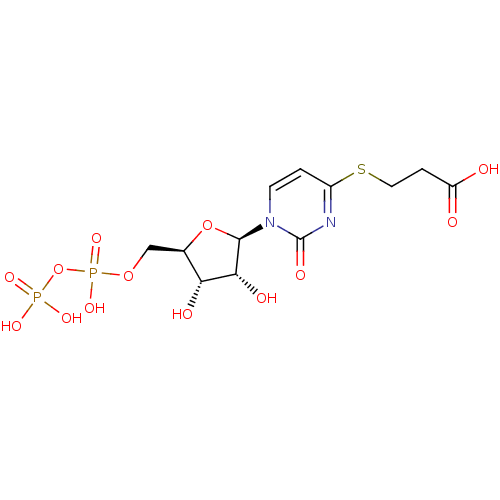 ((2R,3R,4S,5R)-3-(1-(3,4-dihydroxy-5-(diphosphorylo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194160 ((2R,3R,4S,5R)-1-(3,4-dihydroxy-5-(diphosphoryloxym...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 4 (Homo sapiens (Human)) | BDBM50194161 ((2R,3R,5S)-1-(5-(diphosphoryloxymethyl)-3-hydroxyt...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y4 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194159 ((2R,3R,4S,5R)-1-(3,4-dihydroxy-5-(diphosphoryloxym...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194162 (3-methyl-1-beta-D-ribofuranosylpyrimidine-2,4-dion...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194163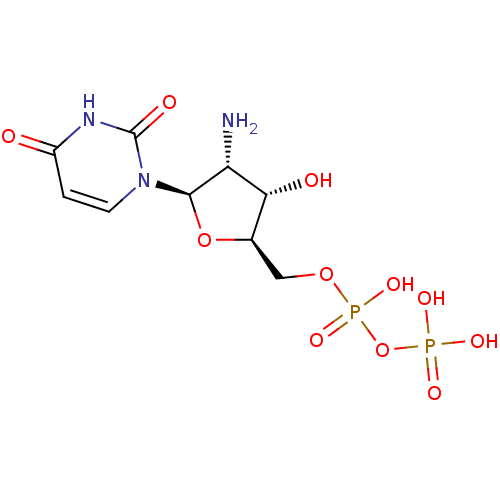 ((2R,3R,4S,5R)-1-(3-amino-5-(diphosphoryloxymethyl)...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194164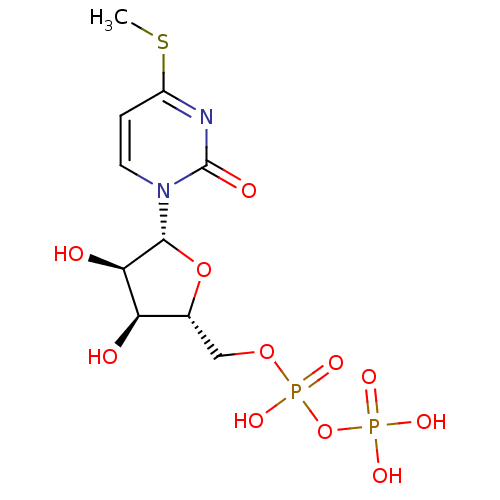 ((2R,3R,4S,5R)-1-(3,4-dihydroxy-5-(diphosphoryloxym...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50194161 ((2R,3R,5S)-1-(5-(diphosphoryloxymethyl)-3-hydroxyt...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 810 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y2 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194167 ((2R,3R,4S,5R)-4-(1-(3,4-dihydroxy-5-(diphosphorylo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194166 ((2R,3R,4S,5R)-1-(3-azido-5-(diphosphoryloxymethyl)...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194165 ((2R,3R,4S,5R)-1-(3,4-dihydroxy-5-(diphosphoryloxym...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50118239 (CHEMBL130266 | UDP | Uridine diphosphate | uridine...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||