

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM78957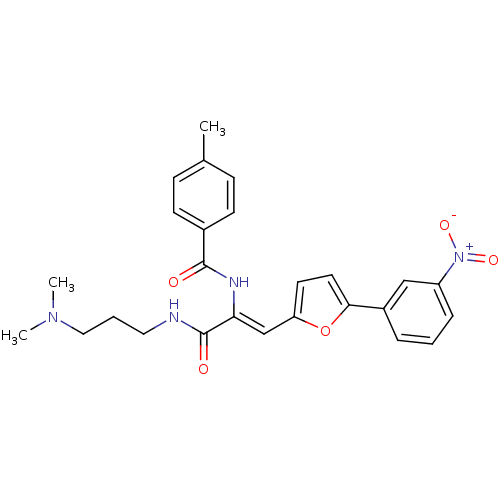 (MLS001163269 | N-[(Z)-1-[3-(dimethylamino)propylca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus recombinant 3C-like protease expressed in Escherichia coli BL21(DE3) by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 3088-91 (2011) Article DOI: 10.1016/j.bmcl.2011.03.034 BindingDB Entry DOI: 10.7270/Q2CC1112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM53105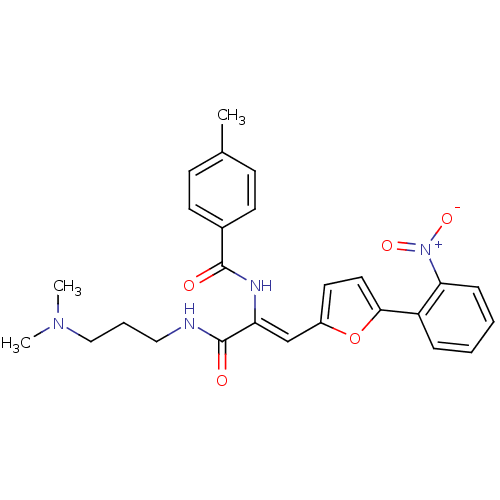 (CHEMBL1315054 | MLS001164598 | N-[(Z)-1-[3-(dimeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chonnam National University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus recombinant 3C-like protease expressed in Escherichia coli BL21(DE3) by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 21: 3088-91 (2011) Article DOI: 10.1016/j.bmcl.2011.03.034 BindingDB Entry DOI: 10.7270/Q2CC1112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50384024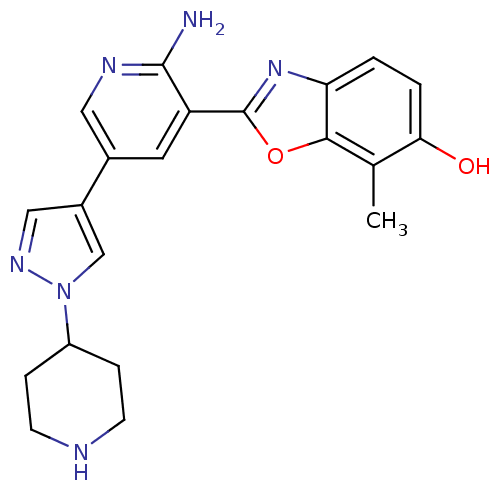 (CHEMBL2032280 | CHEMBL2079349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met by time resolved-fluorescence resonance energy transfer analysis | Bioorg Med Chem Lett 22: 4044-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.083 BindingDB Entry DOI: 10.7270/Q2FF3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50384038 (CHEMBL2032155) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant FLT3 by time resolved-fluorescence resonance energy transfer analysis | Bioorg Med Chem Lett 22: 4044-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.083 BindingDB Entry DOI: 10.7270/Q2FF3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50066870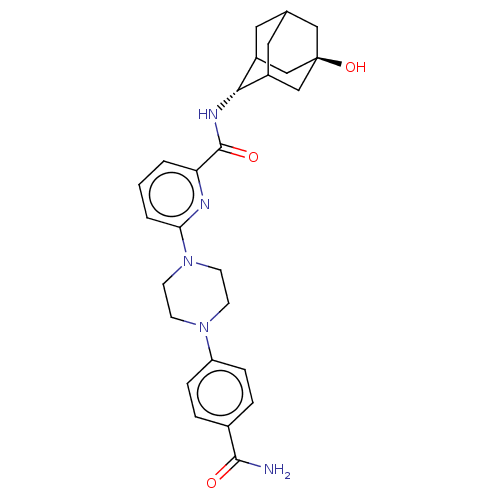 (CHEMBL3401672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells using NADPH assessed as conversion of cortisone to cortisol by cell-based assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50577632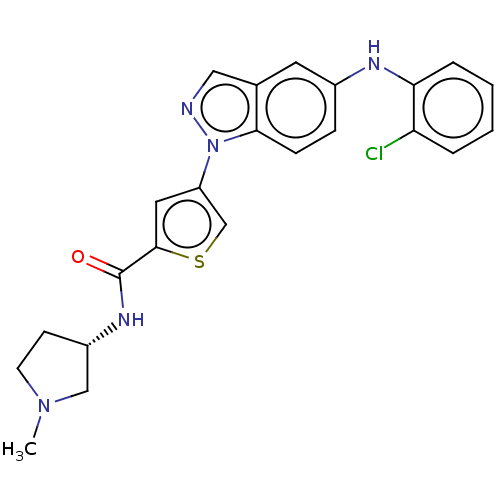 (CHEMBL4875508) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using biotinylated FL-ATF-2 as substrate measured after 22 mins by homogeneous time-resolved fluorescence a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00334 BindingDB Entry DOI: 10.7270/Q2445RBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human Aurora A using H-LRRASLG as substrate after 2 hrs by HotSpot assay in presence of 33P-ATP and 1 uM ATP | Bioorg Med Chem 24: 2114-24 (2016) Article DOI: 10.1016/j.bmc.2016.03.042 BindingDB Entry DOI: 10.7270/Q2ZW1NST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50577630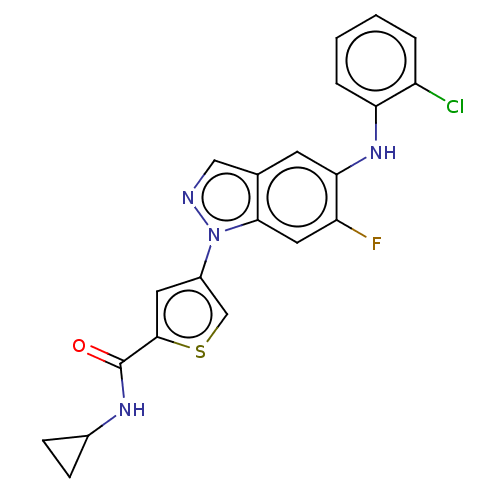 (CHEMBL4861739) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using biotinylated FL-ATF-2 as substrate measured after 22 mins by homogeneous time-resolved fluorescence a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00334 BindingDB Entry DOI: 10.7270/Q2445RBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of human Aurora B using H-LRRASLG as substrate after 2 hrs by HotSpot assay in presence of 33P-ATP and 1 uM ATP | Bioorg Med Chem 24: 2114-24 (2016) Article DOI: 10.1016/j.bmc.2016.03.042 BindingDB Entry DOI: 10.7270/Q2ZW1NST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50032821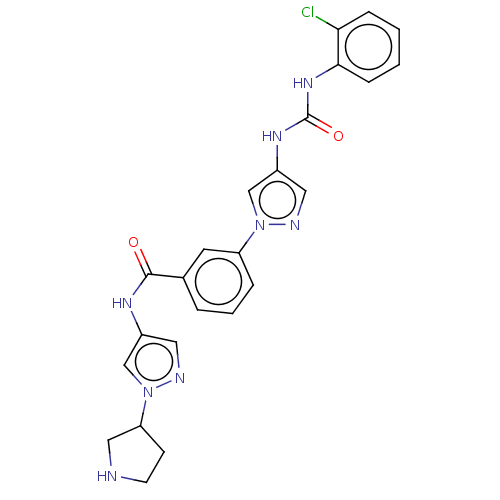 (CHEMBL3355178) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK3alpha1 (unknown origin) using biotinylated FL-ATF-2 as substrate measured after 22 mins by homogeneous time-resolved fluorescence a... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00334 BindingDB Entry DOI: 10.7270/Q2445RBQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Inhibition of Aurora B (unknown origin) using [33P]-ATP and 10 uM ATP after 2 hrs | Bioorg Med Chem 22: 4855-66 (2014) Article DOI: 10.1016/j.bmc.2014.06.047 BindingDB Entry DOI: 10.7270/Q2PV6N11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50431696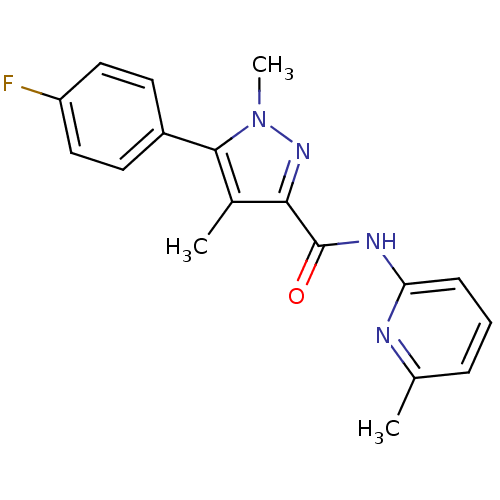 (CHEMBL2349534) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SK Biopharmaceuticals Curated by ChEMBL | Assay Description Negative allosteric modulation of human mGluR5 expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 23: 2134-9 (2013) Article DOI: 10.1016/j.bmcl.2013.01.116 BindingDB Entry DOI: 10.7270/Q20R9QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50458125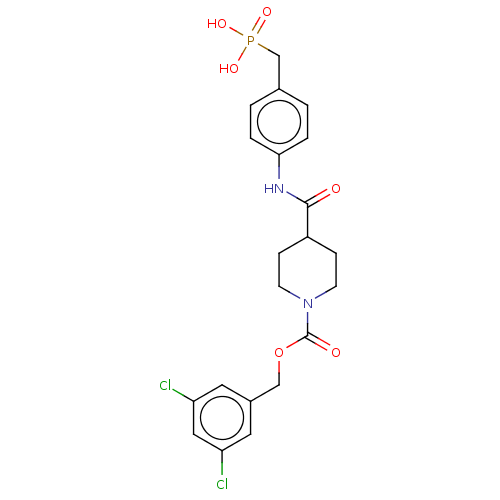 (CHEMBL4217352) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay | Eur J Med Chem 148: 397-409 (2018) Article DOI: 10.1016/j.ejmech.2018.02.049 BindingDB Entry DOI: 10.7270/Q2VH5RGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50458125 (CHEMBL4217352) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of ENPP2 in human A2058 cells using LPC as substrate preincubated for 15 mins followed by substrate addition measured after 3 hrs by LC-MS... | Eur J Med Chem 148: 397-409 (2018) Article DOI: 10.1016/j.ejmech.2018.02.049 BindingDB Entry DOI: 10.7270/Q2VH5RGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50458132 (CHEMBL4205127) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay | Eur J Med Chem 148: 397-409 (2018) Article DOI: 10.1016/j.ejmech.2018.02.049 BindingDB Entry DOI: 10.7270/Q2VH5RGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489343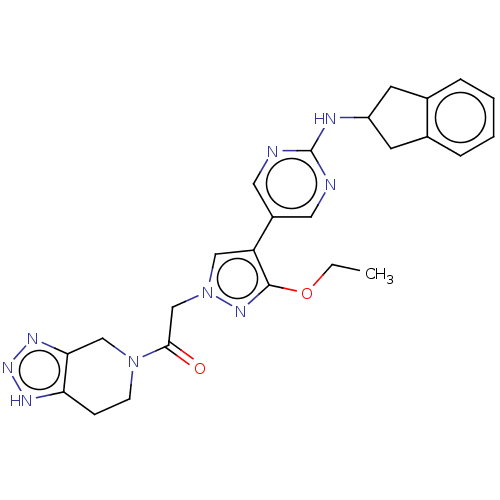 (Example 10-1 | US10961242, Compound 61 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489360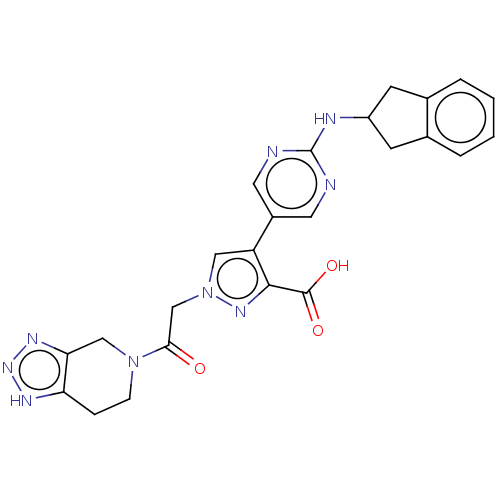 (Example 10-15 | US10961242, Compound 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489343 (Example 10-1 | US10961242, Compound 61 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM582922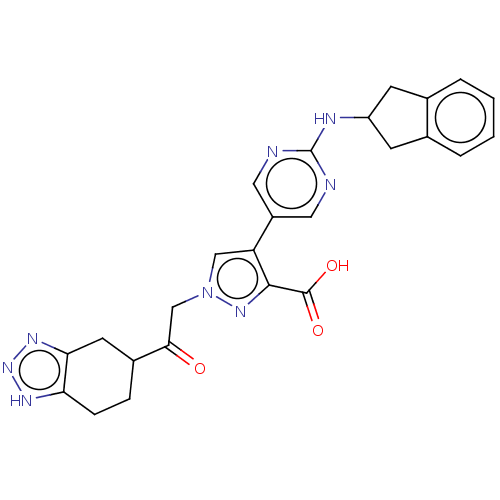 (Acid | US11548883, Compound 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50066894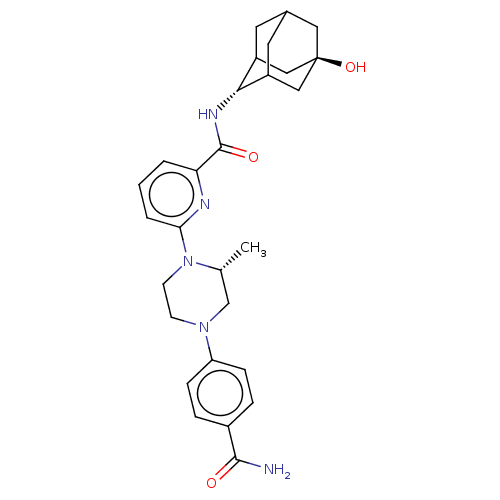 (CHEMBL3401683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells using NADPH assessed as conversion of cortisone to cortisol by cell-based assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489368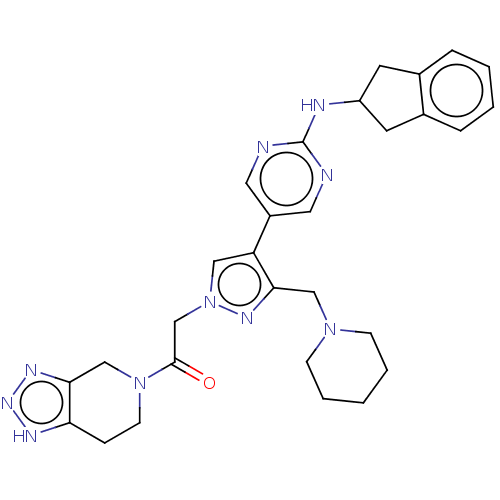 (Example 12-2 | US10961242, Compound 81 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489369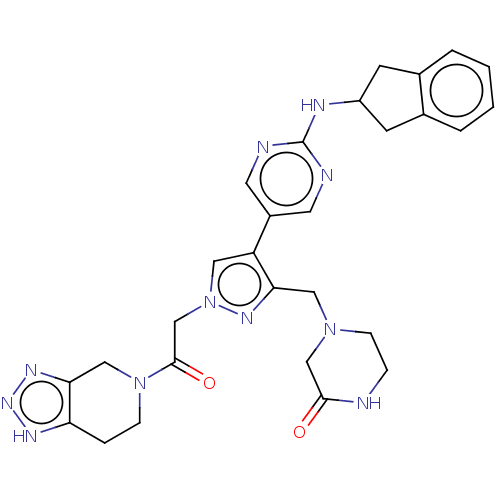 (Example 12-3 | US10961242, Compound 82 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489367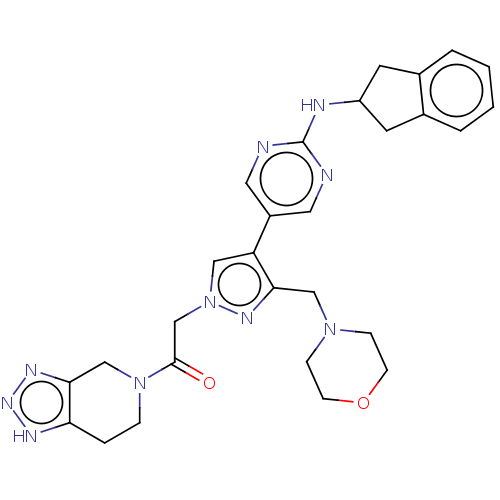 (Example 12-1 | US10961242, Compound 80 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489347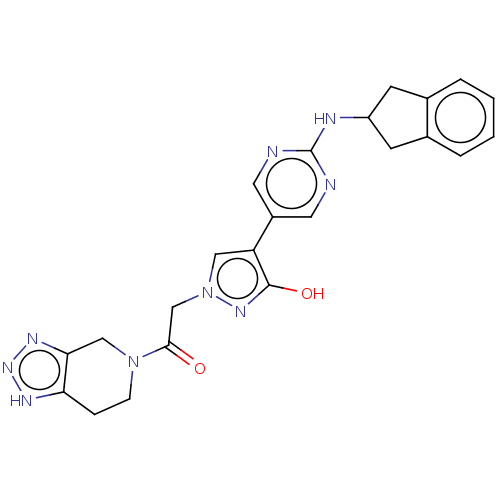 (Example 10-5 | US10961242, Compound 65 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489369 (Example 12-3 | US10961242, Compound 82 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489368 (Example 12-2 | US10961242, Compound 81 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489367 (Example 12-1 | US10961242, Compound 80 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489347 (Example 10-5 | US10961242, Compound 65 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50384038 (CHEMBL2032155) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met by time resolved-fluorescence resonance energy transfer analysis | Bioorg Med Chem Lett 22: 4044-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.083 BindingDB Entry DOI: 10.7270/Q2FF3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489366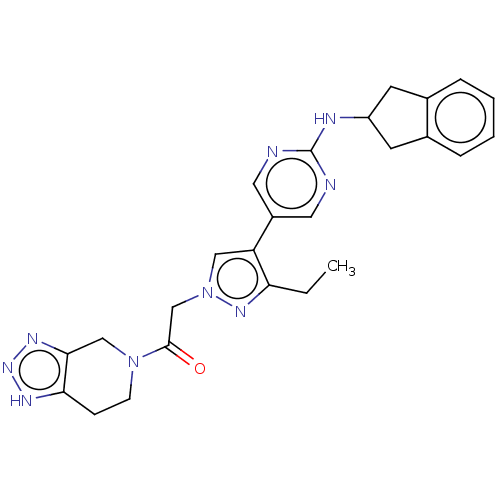 (Example 11-2 | US10961242, Compound 79 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50066871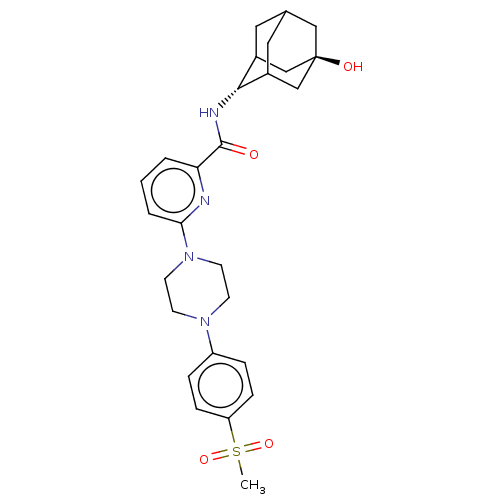 (CHEMBL3401671) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells using NADPH assessed as conversion of cortisone to cortisol by cell-based assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489366 (Example 11-2 | US10961242, Compound 79 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50550263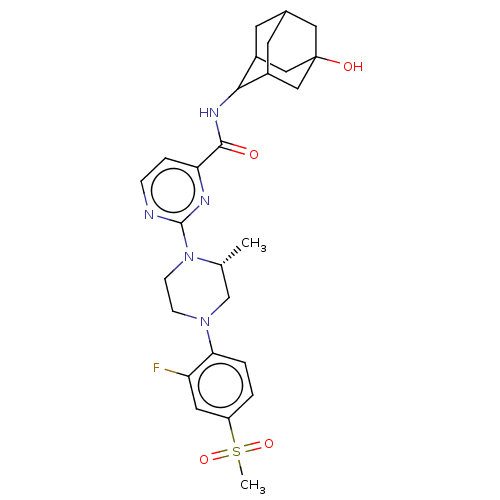 (CHEMBL4748750) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse 11beta-HSD1 expressed in microsomes | Citation and Details BindingDB Entry DOI: 10.7270/Q27P930B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489357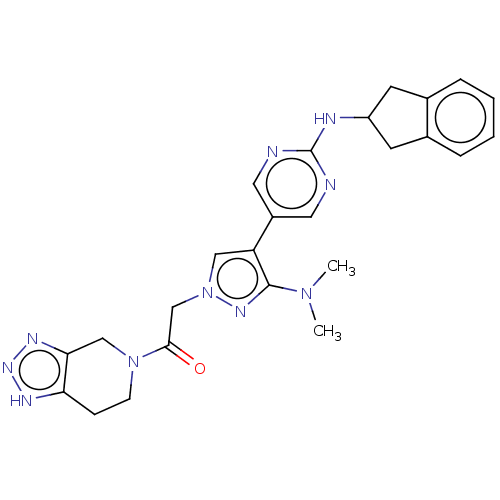 (Example 10-14 | US10961242, Compound 74 | US115488...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489357 (Example 10-14 | US10961242, Compound 74 | US115488...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50458135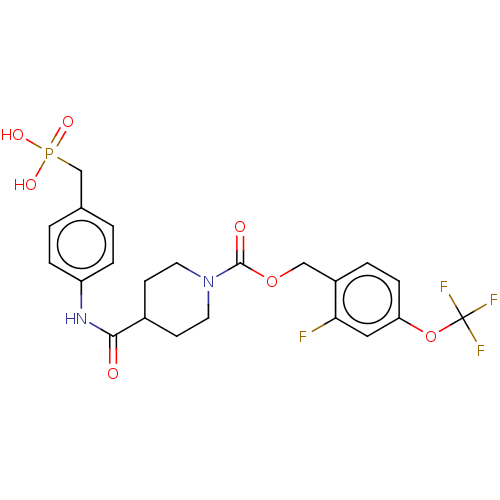 (CHEMBL4214731) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay | Eur J Med Chem 148: 397-409 (2018) Article DOI: 10.1016/j.ejmech.2018.02.049 BindingDB Entry DOI: 10.7270/Q2VH5RGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50187693 (CHEMBL3186509) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of New Drug Discovery and Development, Chungnam National University, Daejeon 305-764, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human ATX beta expressed in HEK293 cells using LPC as substrate measured after 30 mins by LC-MS/MS analysis | Bioorg Med Chem Lett 27: 4156-4164 (2017) Article DOI: 10.1016/j.bmcl.2017.07.022 BindingDB Entry DOI: 10.7270/Q24F1T6B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50060496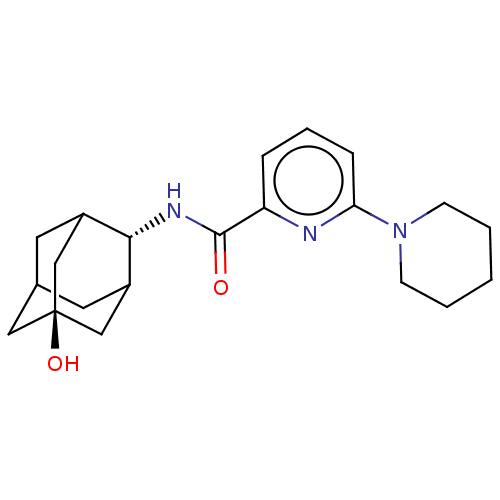 (CHEMBL3394399) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Republic of Korea; Research Institute of Pharmaceutical Science and College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in HEK293 cell microsomal fraction | Bioorg Med Chem Lett 25: 695-700 (2015) Article DOI: 10.1016/j.bmcl.2014.11.074 BindingDB Entry DOI: 10.7270/Q24B32ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50060501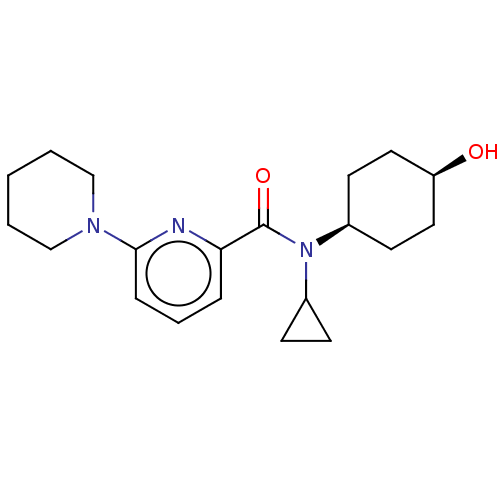 (CHEMBL3394394) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Republic of Korea; Research Institute of Pharmaceutical Science and College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in HEK293 cell microsomal fraction | Bioorg Med Chem Lett 25: 695-700 (2015) Article DOI: 10.1016/j.bmcl.2014.11.074 BindingDB Entry DOI: 10.7270/Q24B32ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489351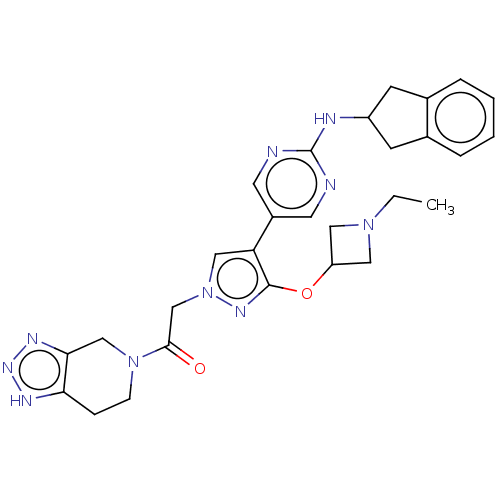 (Example 10-8 | US10961242, Compound 68 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50066887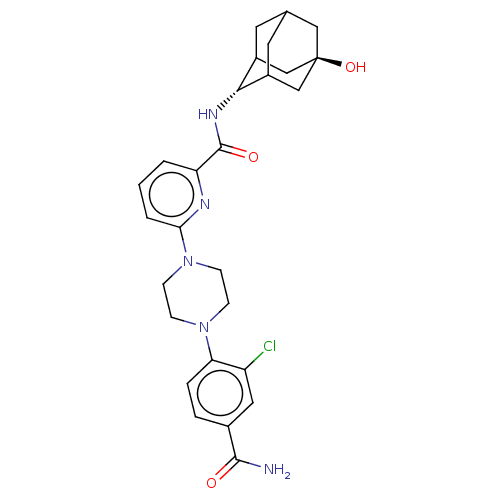 (CHEMBL3401676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells using NADPH assessed as conversion of cortisone to cortisol by cell-based assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50458131 (CHEMBL4212398) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay | Eur J Med Chem 148: 397-409 (2018) Article DOI: 10.1016/j.ejmech.2018.02.049 BindingDB Entry DOI: 10.7270/Q2VH5RGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489351 (Example 10-8 | US10961242, Compound 68 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50066892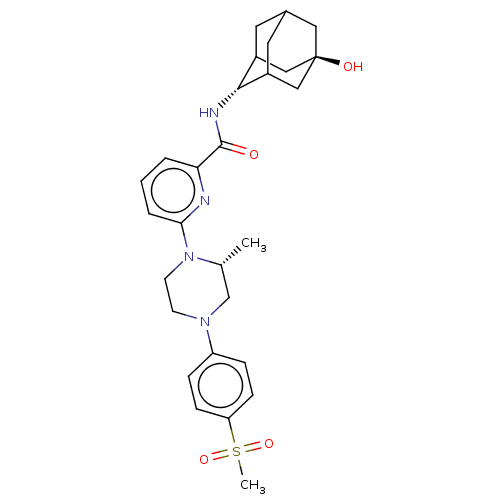 (CHEMBL3401681) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 using microsomal fraction and NADPH assessed as conversion of cortisone to cortisol by biochemical enzyme assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50458127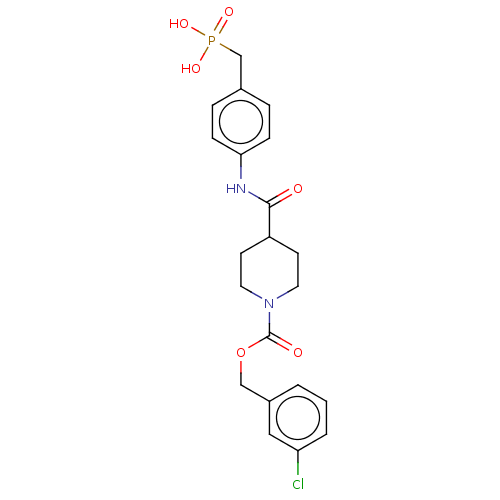 (CHEMBL4211269) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of human ENPP2 using FS3 as substrate measured every 5 mins for 30 mins by fluorescence assay | Eur J Med Chem 148: 397-409 (2018) Article DOI: 10.1016/j.ejmech.2018.02.049 BindingDB Entry DOI: 10.7270/Q2VH5RGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489342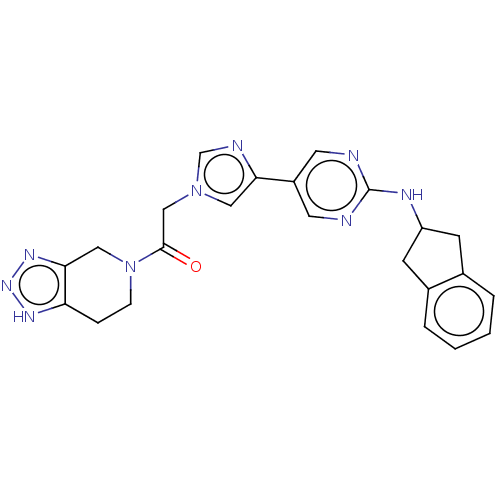 (Example 9 | US10961242, Compound 60) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM582907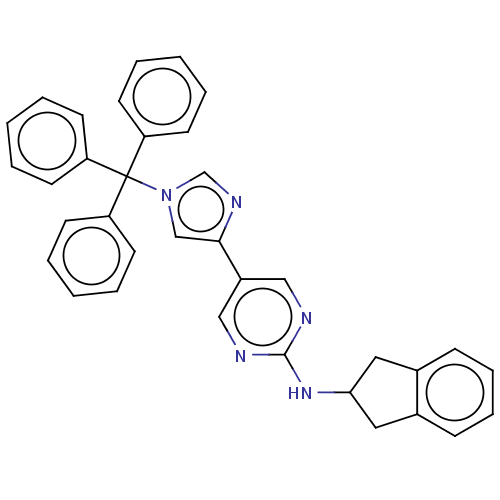 (2-(4-{2-[(2,3-dihydro-1H-inden-2-yl)amino]pyrimidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489348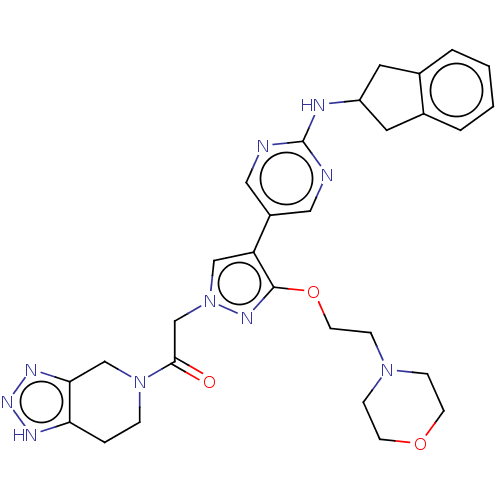 (Example 10-6 | US10961242, Compound 66 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50060491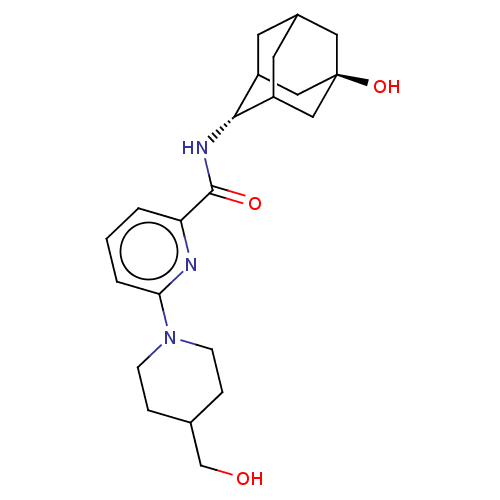 (CHEMBL3394404) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Republic of Korea; Research Institute of Pharmaceutical Science and College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in HEK293 cell microsomal fraction | Bioorg Med Chem Lett 25: 695-700 (2015) Article DOI: 10.1016/j.bmcl.2014.11.074 BindingDB Entry DOI: 10.7270/Q24B32ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489348 (Example 10-6 | US10961242, Compound 66 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1720 total ) | Next | Last >> |