 Found 52 hits with Last Name = 'wietrzyk' and Initial = 'j'
Found 52 hits with Last Name = 'wietrzyk' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50604012

(CHEMBL5199004)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cn(nn1)-c1cc(F)cc(F)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM13058
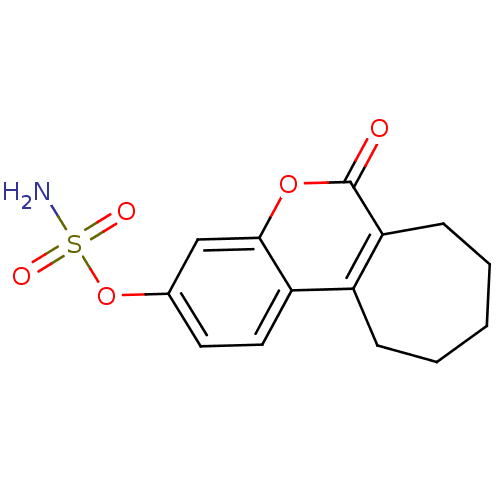
(6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...)Show InChI InChI=1S/C14H15NO5S/c15-21(17,18)20-9-6-7-11-10-4-2-1-3-5-12(10)14(16)19-13(11)8-9/h6-8H,1-5H2,(H2,15,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50604011
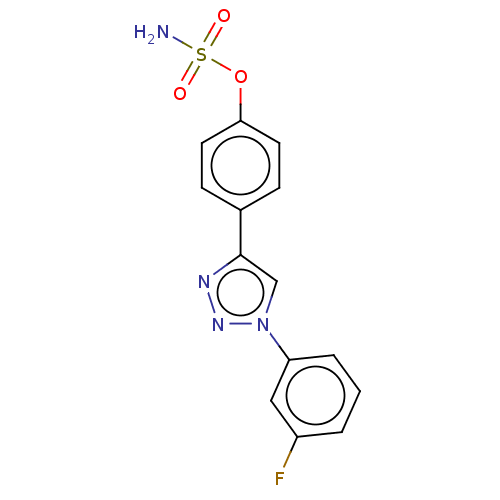
(CHEMBL5205557)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cn(nn1)-c1cccc(F)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50604009
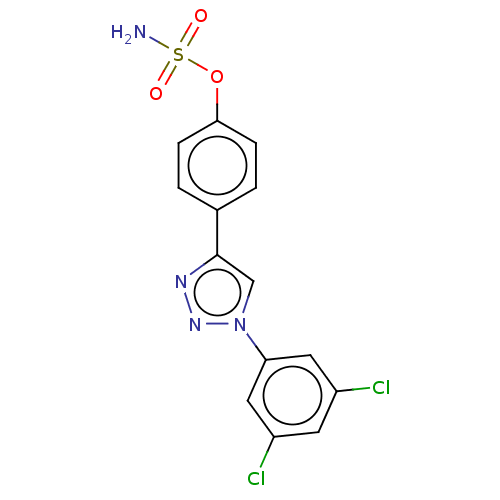
(CHEMBL5170413)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cn(nn1)-c1cc(Cl)cc(Cl)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50604008
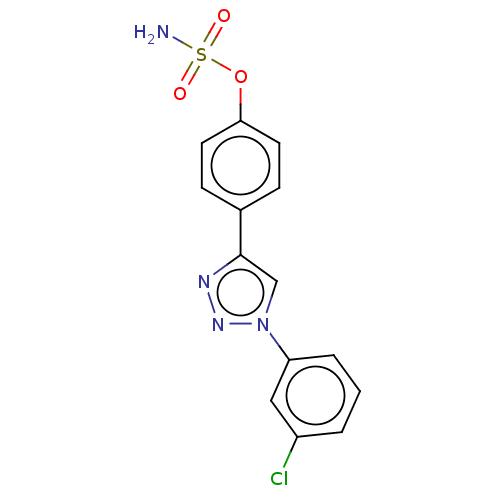
(CHEMBL5179747)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cn(nn1)-c1cccc(Cl)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50604010
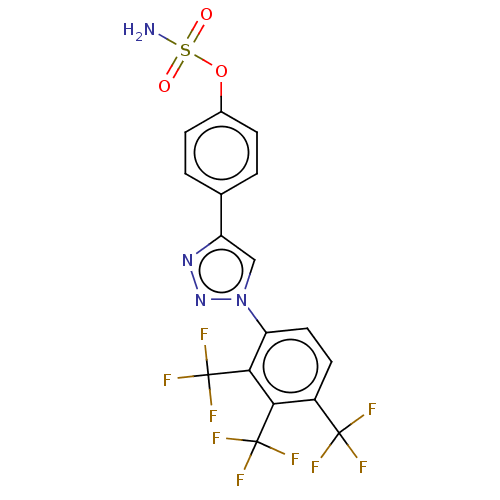
(CHEMBL5169672)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cn(nn1)-c1ccc(c(c1C(F)(F)F)C(F)(F)F)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50604012

(CHEMBL5199004)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cn(nn1)-c1cc(F)cc(F)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50051829
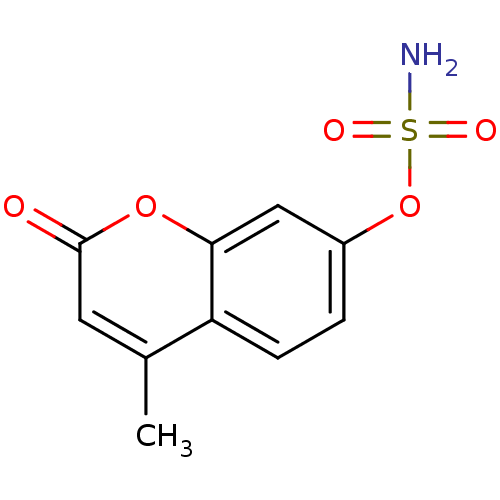
(4-methyl-2-oxo-2H-chromen-7-yl sulfamate | CHEMBL1...)Show InChI InChI=1S/C10H9NO5S/c1-6-4-10(12)15-9-5-7(2-3-8(6)9)16-17(11,13)14/h2-5H,1H3,(H2,11,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513186
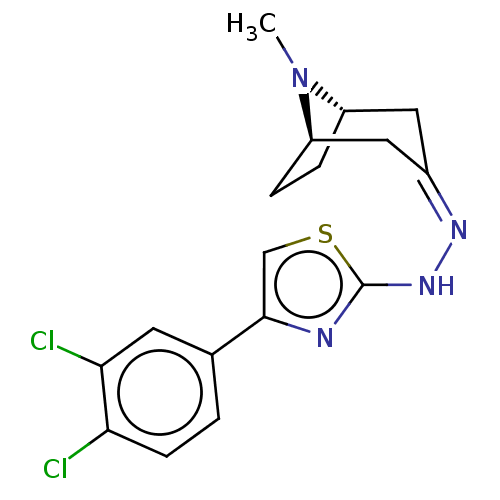
(CHEMBL4588381)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(Cl)c(Cl)c1)N2C |r,TLB:9:7:24:2.3| Show InChI InChI=1S/C17H18Cl2N4S/c1-23-12-3-4-13(23)8-11(7-12)21-22-17-20-16(9-24-17)10-2-5-14(18)15(19)6-10/h2,5-6,9,12-13H,3-4,7-8H2,1H3,(H,20,22)/b21-11-/t12-,13+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513187
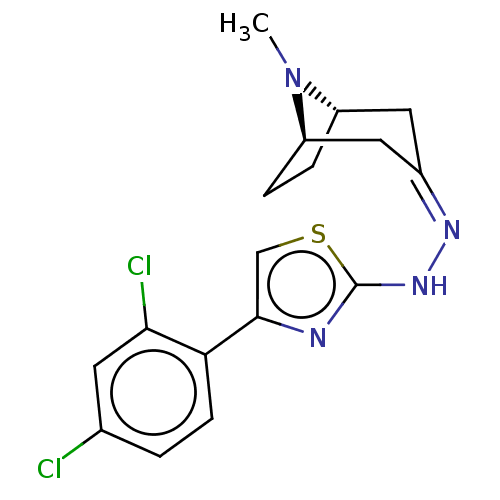
(CHEMBL4583240)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(Cl)cc1Cl)N2C |r,TLB:9:7:24:2.3| Show InChI InChI=1S/C17H18Cl2N4S/c1-23-12-3-4-13(23)8-11(7-12)21-22-17-20-16(9-24-17)14-5-2-10(18)6-15(14)19/h2,5-6,9,12-13H,3-4,7-8H2,1H3,(H,20,22)/b21-11-/t12-,13+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 10 hrs measured every 6 mins by fluor... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534075

(CHEMBL4483393)Show SMILES CCOC(=O)C1(Cc2cc(OCc3ccccc3)cc(OCc3ccccc3)c2C=N1)C(=O)OCC |c:32| Show InChI InChI=1S/C29H29NO6/c1-3-33-27(31)29(28(32)34-4-2)17-23-15-24(35-19-21-11-7-5-8-12-21)16-26(25(23)18-30-29)36-20-22-13-9-6-10-14-22/h5-16,18H,3-4,17,19-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534075

(CHEMBL4483393)Show SMILES CCOC(=O)C1(Cc2cc(OCc3ccccc3)cc(OCc3ccccc3)c2C=N1)C(=O)OCC |c:32| Show InChI InChI=1S/C29H29NO6/c1-3-33-27(31)29(28(32)34-4-2)17-23-15-24(35-19-21-11-7-5-8-12-21)16-26(25(23)18-30-29)36-20-22-13-9-6-10-14-22/h5-16,18H,3-4,17,19-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 10 hrs measured every 6 mins by fluor... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513185
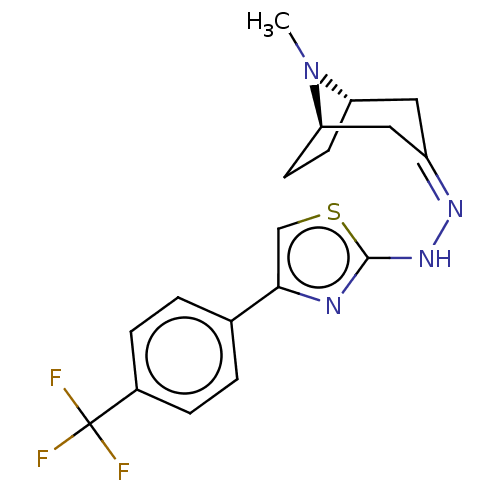
(CHEMBL4463818)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(cc1)C(F)(F)F)N2C |r,TLB:9:7:26:2.3| Show InChI InChI=1S/C18H19F3N4S/c1-25-14-6-7-15(25)9-13(8-14)23-24-17-22-16(10-26-17)11-2-4-12(5-3-11)18(19,20)21/h2-5,10,14-15H,6-9H2,1H3,(H,22,24)/b23-13-/t14-,15+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534077
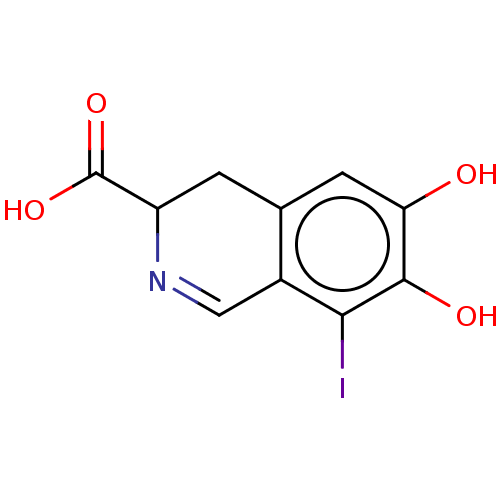
(CHEMBL4464704)Show InChI InChI=1S/C10H8INO4/c11-8-5-3-12-6(10(15)16)1-4(5)2-7(13)9(8)14/h2-3,6,13-14H,1H2,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534070
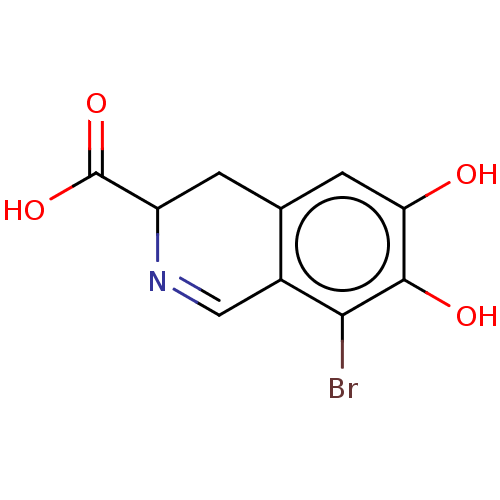
(CHEMBL4454667)Show InChI InChI=1S/C10H8BrNO4/c11-8-5-3-12-6(10(15)16)1-4(5)2-7(13)9(8)14/h2-3,6,13-14H,1H2,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534074
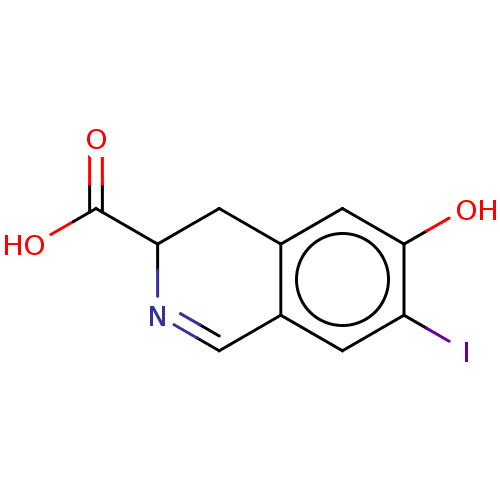
(CHEMBL4468968)Show InChI InChI=1S/C10H8INO3/c11-7-1-6-4-12-8(10(14)15)2-5(6)3-9(7)13/h1,3-4,8,13H,2H2,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50031467

(5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...)Show InChI InChI=1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513188
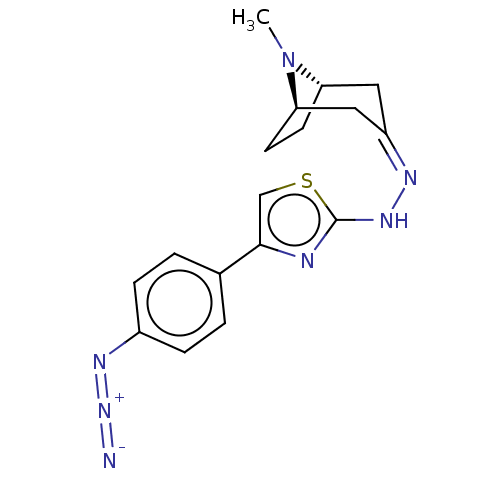
(CHEMBL4453112)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(cc1)N=[N+]=[N-])N2C |r,TLB:9:7:25:2.3| Show InChI InChI=1S/C17H19N7S/c1-24-14-6-7-15(24)9-13(8-14)20-22-17-19-16(10-25-17)11-2-4-12(5-3-11)21-23-18/h2-5,10,14-15H,6-9H2,1H3,(H,19,22)/b20-13-/t14-,15+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534069
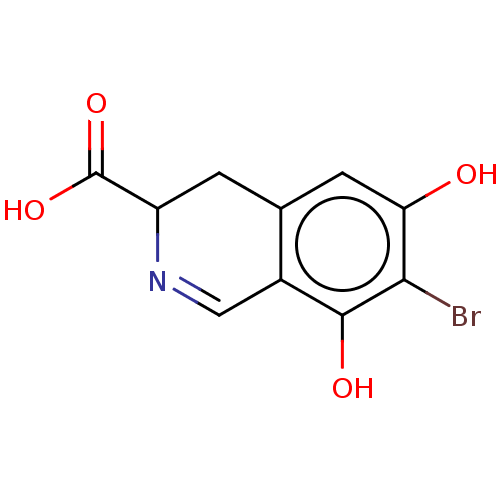
(CHEMBL4473220)Show InChI InChI=1S/C10H8BrNO4/c11-8-7(13)2-4-1-6(10(15)16)12-3-5(4)9(8)14/h2-3,6,13-14H,1H2,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513183
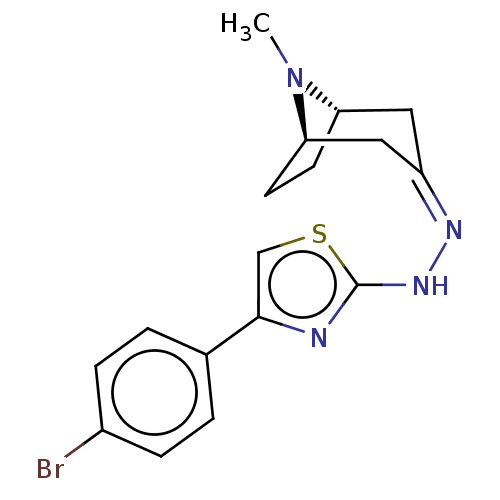
(CHEMBL4473661)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(Br)cc1)N2C |r,TLB:9:7:23:2.3| Show InChI InChI=1S/C17H19BrN4S/c1-22-14-6-7-15(22)9-13(8-14)20-21-17-19-16(10-23-17)11-2-4-12(18)5-3-11/h2-5,10,14-15H,6-9H2,1H3,(H,19,21)/b20-13-/t14-,15+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513190

(CHEMBL4438867)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(C)cc1)N2C |r,TLB:9:7:23:2.3| Show InChI InChI=1S/C18H22N4S/c1-12-3-5-13(6-4-12)17-11-23-18(19-17)21-20-14-9-15-7-8-16(10-14)22(15)2/h3-6,11,15-16H,7-10H2,1-2H3,(H,19,21)/b20-14-/t15-,16+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534072

(CHEMBL4455383)Show InChI InChI=1S/C10H9NO4/c12-8-2-5-1-7(10(14)15)11-4-6(5)3-9(8)13/h2-4,7,12-13H,1H2,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513189
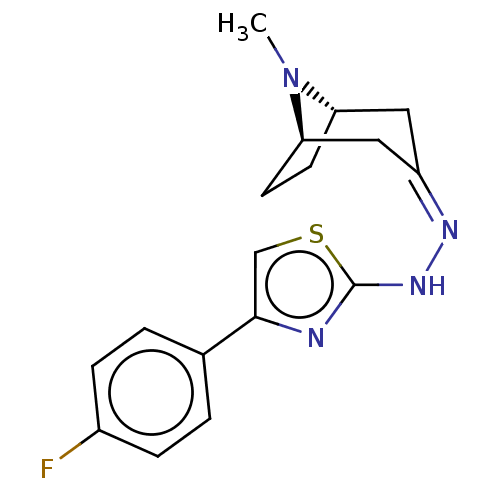
(CHEMBL4555931)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(F)cc1)N2C |r,TLB:9:7:23:2.3| Show InChI InChI=1S/C17H19FN4S/c1-22-14-6-7-15(22)9-13(8-14)20-21-17-19-16(10-23-17)11-2-4-12(18)5-3-11/h2-5,10,14-15H,6-9H2,1H3,(H,19,21)/b20-13-/t14-,15+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513184
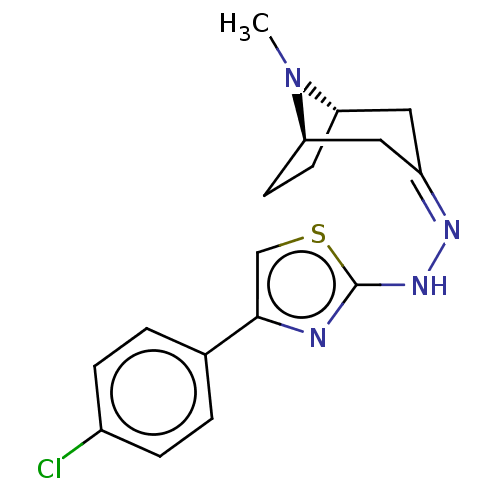
(CHEMBL4456453)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(Cl)cc1)N2C |r,TLB:9:7:23:2.3| Show InChI InChI=1S/C17H19ClN4S/c1-22-14-6-7-15(22)9-13(8-14)20-21-17-19-16(10-23-17)11-2-4-12(18)5-3-11/h2-5,10,14-15H,6-9H2,1H3,(H,19,21)/b20-13-/t14-,15+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534078

(CHEMBL4557950)Show InChI InChI=1S/C10H9NO5/c12-7-1-5-3-10(16,9(14)15)11-4-6(5)2-8(7)13/h1-2,4,12-13,16H,3H2,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534081

(CHEMBL4460083)Show InChI InChI=1S/C11H11NO4/c13-9-3-6-1-2-8(11(15)16)12-5-7(6)4-10(9)14/h3-5,8,13-14H,1-2H2,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138810
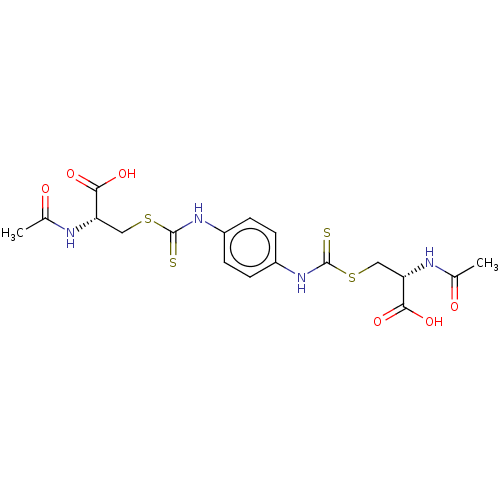
(CHEMBL3752558)Show SMILES CC(=O)N[C@@H](CSC(=S)Nc1ccc(NC(=S)SC[C@H](NC(C)=O)C(O)=O)cc1)C(O)=O |r| Show InChI InChI=1S/C18H22N4O6S4/c1-9(23)19-13(15(25)26)7-31-17(29)21-11-3-5-12(6-4-11)22-18(30)32-8-14(16(27)28)20-10(2)24/h3-6,13-14H,7-8H2,1-2H3,(H,19,23)(H,20,24)(H,21,29)(H,22,30)(H,25,26)(H,27,28)/t13-,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534071
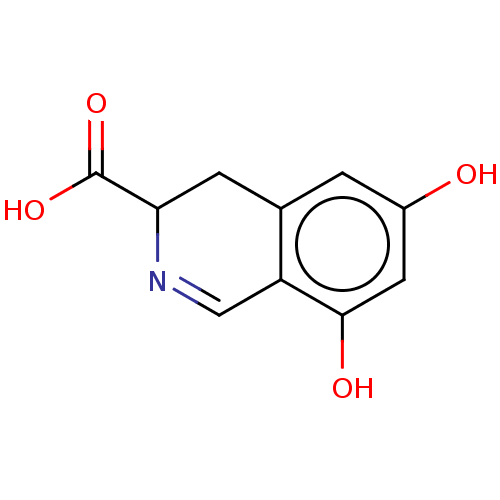
(CHEMBL4563759)Show InChI InChI=1S/C10H9NO4/c12-6-1-5-2-8(10(14)15)11-4-7(5)9(13)3-6/h1,3-4,8,12-13H,2H2,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50351096
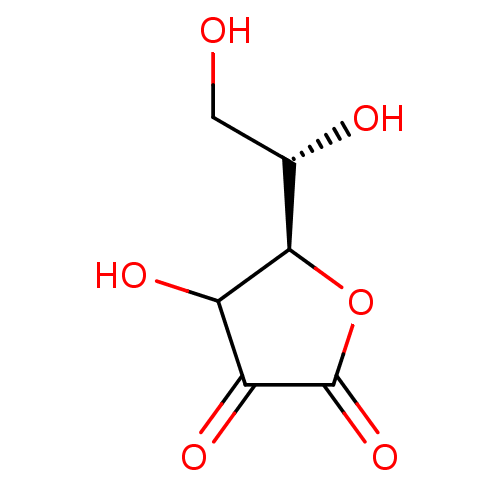
(ASCORBIC ACID)Show InChI InChI=1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2-3,5,7-9H,1H2/t2-,3?,5+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534080
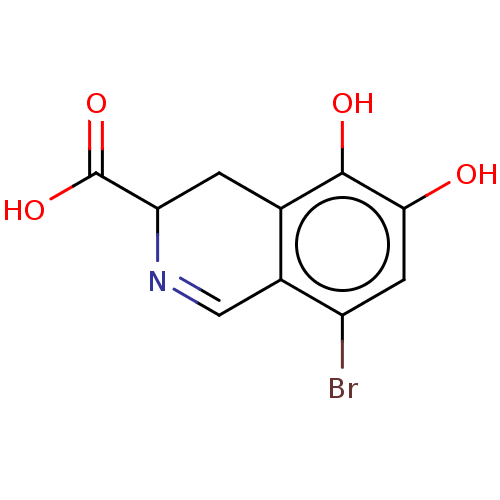
(CHEMBL4576374)Show InChI InChI=1S/C10H8BrNO4/c11-6-2-8(13)9(14)4-1-7(10(15)16)12-3-5(4)6/h2-3,7,13-14H,1H2,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138812
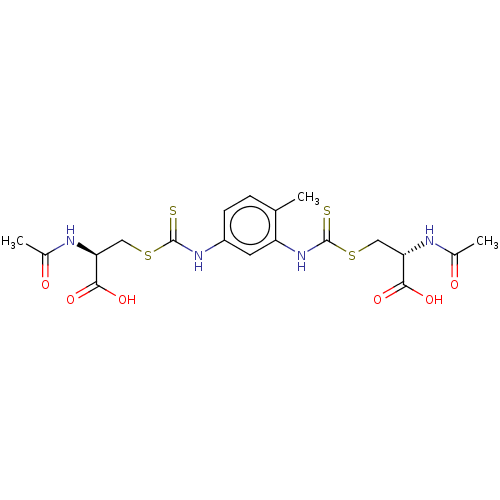
(CHEMBL3754172)Show SMILES CC(=O)N[C@@H](CSC(=S)Nc1ccc(C)c(NC(=S)SC[C@H](NC(C)=O)C(O)=O)c1)C(O)=O |r| Show InChI InChI=1S/C19H24N4O6S4/c1-9-4-5-12(22-18(30)32-7-14(16(26)27)20-10(2)24)6-13(9)23-19(31)33-8-15(17(28)29)21-11(3)25/h4-6,14-15H,7-8H2,1-3H3,(H,20,24)(H,21,25)(H,22,30)(H,23,31)(H,26,27)(H,28,29)/t14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534073

(CHEMBL4537106)Show InChI InChI=1S/C10H9NO5/c12-7-2-4-1-6(10(15)16)11-3-5(4)8(13)9(7)14/h2-3,6,12-14H,1H2,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138811
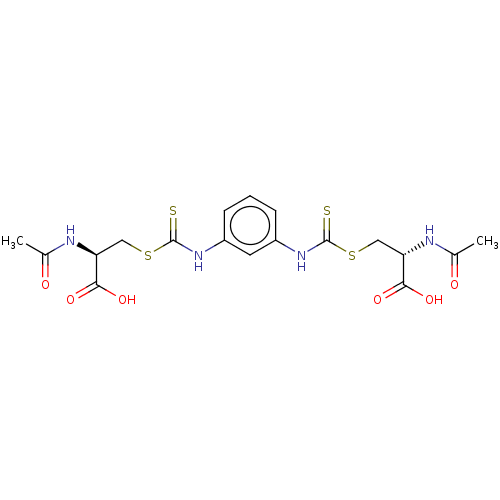
(CHEMBL3753294)Show SMILES CC(=O)N[C@@H](CSC(=S)Nc1cccc(NC(=S)SC[C@H](NC(C)=O)C(O)=O)c1)C(O)=O |r| Show InChI InChI=1S/C18H22N4O6S4/c1-9(23)19-13(15(25)26)7-31-17(29)21-11-4-3-5-12(6-11)22-18(30)32-8-14(16(27)28)20-10(2)24/h3-6,13-14H,7-8H2,1-2H3,(H,19,23)(H,20,24)(H,21,29)(H,22,30)(H,25,26)(H,27,28)/t13-,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534076
(CHEMBL4435767)Show SMILES COc1cc2CCC(N=Cc2cc1OC)(C(=O)OCc1ccccc1)C(=O)OCc1ccccc1 |c:8| Show InChI InChI=1S/C28H27NO6/c1-32-24-15-22-13-14-28(29-17-23(22)16-25(24)33-2,26(30)34-18-20-9-5-3-6-10-20)27(31)35-19-21-11-7-4-8-12-21/h3-12,15-17H,13-14,18-19H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.05E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
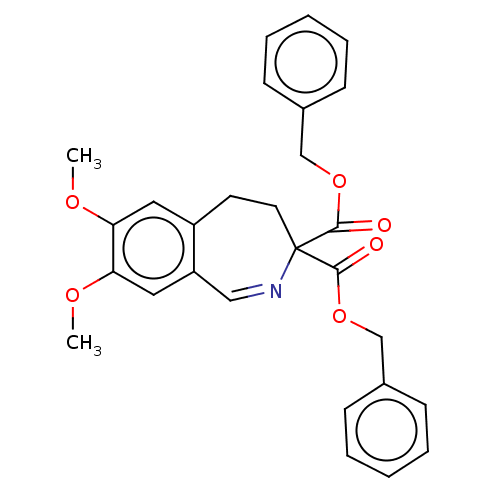
Cytosol aminopeptidase
(Sus scrofa) | BDBM50534079
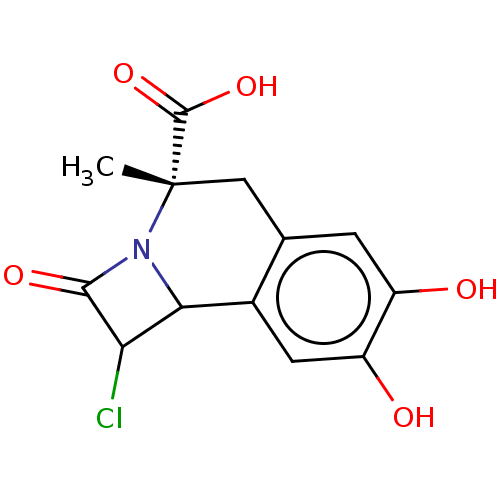
(CHEMBL4456116)Show SMILES C[C@]1(Cc2cc(O)c(O)cc2C2C(Cl)C(=O)N12)C(O)=O |r| Show InChI InChI=1S/C13H12ClNO5/c1-13(12(19)20)4-5-2-7(16)8(17)3-6(5)10-9(14)11(18)15(10)13/h2-3,9-10,16-17H,4H2,1H3,(H,19,20)/t9?,10?,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.11E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Public Health-National Institute of Hygiene
Curated by ChEMBL
| Assay Description
Inhibition of porcine kidney microsomal LAP using l-leucine-7-amido-4-methylcoumarin as substrate incubated for 60 mins measured every 3 mins by fluo... |
Bioorg Med Chem 24: 5302-5314 (2016)
Article DOI: 10.1016/j.bmc.2016.08.054
BindingDB Entry DOI: 10.7270/Q2NK3JHV |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138807
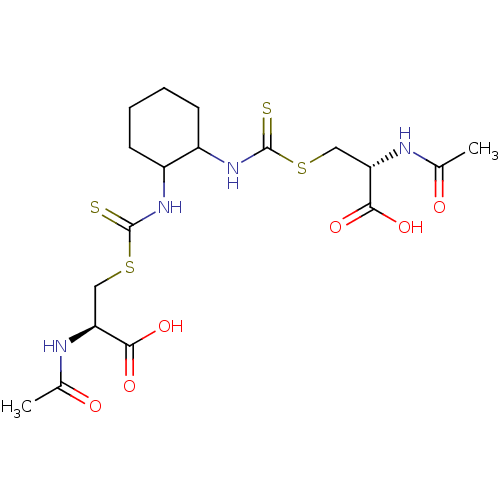
(CHEMBL3752979)Show SMILES CC(=O)N[C@@H](CSC(=S)NC1CCCCC1NC(=S)SC[C@H](NC(C)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C18H28N4O6S4/c1-9(23)19-13(15(25)26)7-31-17(29)21-11-5-3-4-6-12(11)22-18(30)32-8-14(16(27)28)20-10(2)24/h11-14H,3-8H2,1-2H3,(H,19,23)(H,20,24)(H,21,29)(H,22,30)(H,25,26)(H,27,28)/t11?,12?,13-,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138804
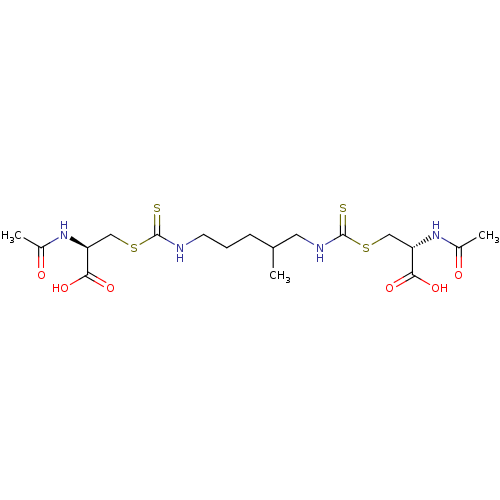
(CHEMBL3752524)Show SMILES CC(CCCNC(=S)SC[C@H](NC(C)=O)C(O)=O)CNC(=S)SC[C@H](NC(C)=O)C(O)=O |r| Show InChI InChI=1S/C18H30N4O6S4/c1-10(7-20-18(30)32-9-14(16(27)28)22-12(3)24)5-4-6-19-17(29)31-8-13(15(25)26)21-11(2)23/h10,13-14H,4-9H2,1-3H3,(H,19,29)(H,20,30)(H,21,23)(H,22,24)(H,25,26)(H,27,28)/t10?,13-,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138803
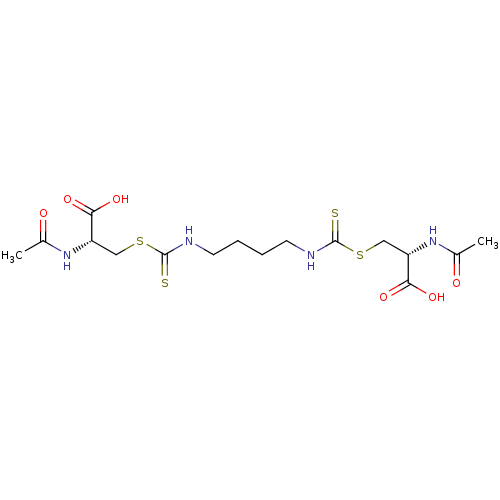
(CHEMBL3752412)Show SMILES CC(=O)N[C@@H](CSC(=S)NCCCCNC(=S)SC[C@H](NC(C)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C16H26N4O6S4/c1-9(21)19-11(13(23)24)7-29-15(27)17-5-3-4-6-18-16(28)30-8-12(14(25)26)20-10(2)22/h11-12H,3-8H2,1-2H3,(H,17,27)(H,18,28)(H,19,21)(H,20,22)(H,23,24)(H,25,26)/t11-,12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.33E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138809
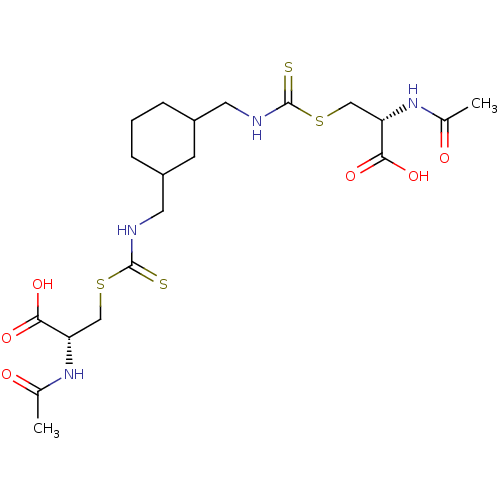
(CHEMBL3754062)Show SMILES CC(=O)N[C@@H](CSC(=S)NCC1CCCC(CNC(=S)SC[C@H](NC(C)=O)C(O)=O)C1)C(O)=O |r| Show InChI InChI=1S/C20H32N4O6S4/c1-11(25)23-15(17(27)28)9-33-19(31)21-7-13-4-3-5-14(6-13)8-22-20(32)34-10-16(18(29)30)24-12(2)26/h13-16H,3-10H2,1-2H3,(H,21,31)(H,22,32)(H,23,25)(H,24,26)(H,27,28)(H,29,30)/t13?,14?,15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.68E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138817
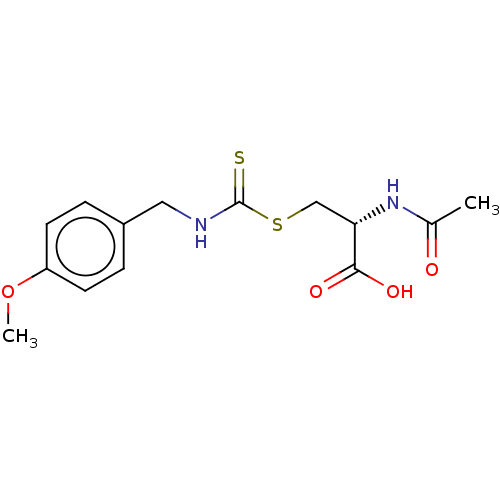
(CHEMBL3752507)Show SMILES COc1ccc(CNC(=S)SC[C@H](NC(C)=O)C(O)=O)cc1 |r| Show InChI InChI=1S/C14H18N2O4S2/c1-9(17)16-12(13(18)19)8-22-14(21)15-7-10-3-5-11(20-2)6-4-10/h3-6,12H,7-8H2,1-2H3,(H,15,21)(H,16,17)(H,18,19)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.94E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50420190
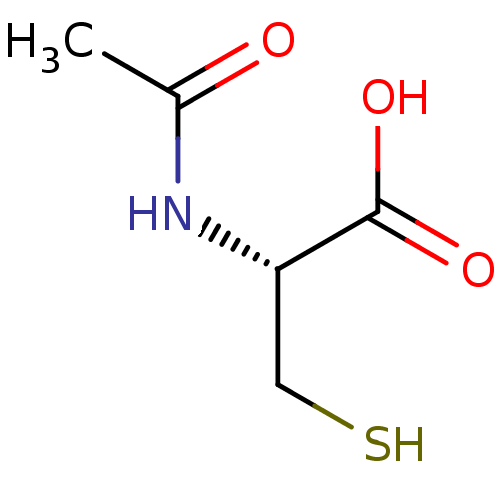
(5052 | ACETYLCYSTEINE)Show InChI InChI=1S/C5H9NO3S/c1-3(7)6-4(2-10)5(8)9/h4,10H,2H2,1H3,(H,6,7)(H,8,9)/t4-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138814
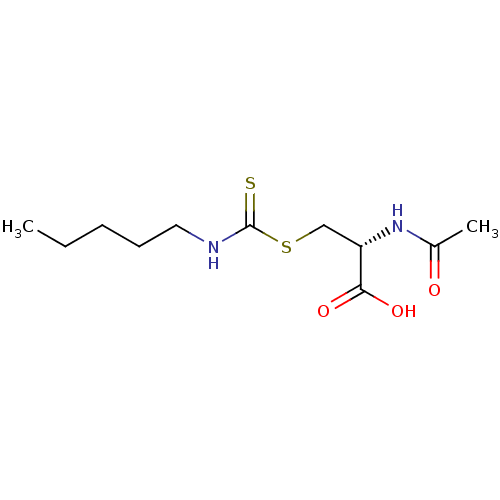
(CHEMBL3752230)Show InChI InChI=1S/C11H20N2O3S2/c1-3-4-5-6-12-11(17)18-7-9(10(15)16)13-8(2)14/h9H,3-7H2,1-2H3,(H,12,17)(H,13,14)(H,15,16)/t9-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138815

(CHEMBL3753774)Show InChI InChI=1S/C13H16N2O3S2/c1-9(16)15-11(12(17)18)8-20-13(19)14-7-10-5-3-2-4-6-10/h2-6,11H,7-8H2,1H3,(H,14,19)(H,15,16)(H,17,18)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.44E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
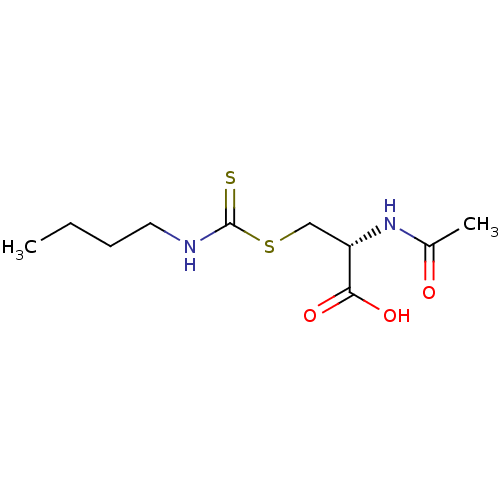
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138816
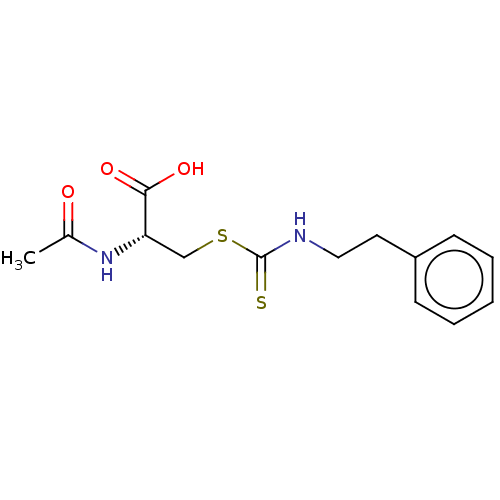
(CHEMBL3752846)Show SMILES CC(=O)N[C@@H](CSC(=S)NCCc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C14H18N2O3S2/c1-10(17)16-12(13(18)19)9-21-14(20)15-8-7-11-5-3-2-4-6-11/h2-6,12H,7-9H2,1H3,(H,15,20)(H,16,17)(H,18,19)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.45E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138813
(CHEMBL3754758)Show InChI InChI=1S/C10H18N2O3S2/c1-3-4-5-11-10(16)17-6-8(9(14)15)12-7(2)13/h8H,3-6H2,1-2H3,(H,11,16)(H,12,13)(H,14,15)/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.46E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138808
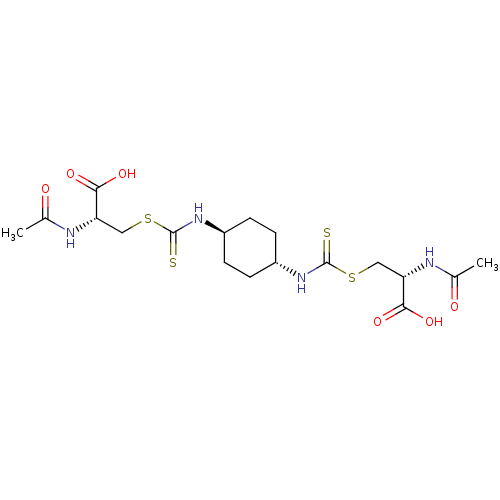
(CHEMBL3754699)Show SMILES CC(=O)N[C@@H](CSC(=S)N[C@H]1CC[C@@H](CC1)NC(=S)SC[C@H](NC(C)=O)C(O)=O)C(O)=O |r,wU:13.16,4.4,wD:10.9,21.21,(-7.73,7.87,;-6.66,8.49,;-6.66,9.72,;-5.33,7.71,;-5.33,6.17,;-3.99,5.4,;-4,3.86,;-2.66,3.08,;-1.6,3.7,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;2.67,-1.54,;4,-.77,;4,.47,;5.34,-1.53,;6.67,-.76,;8.01,-1.53,;9.34,-.76,;10.68,-1.52,;10.68,-2.76,;11.74,-.91,;8.01,-3.07,;6.95,-3.69,;9.08,-3.68,;-6.67,5.41,;-6.67,4.17,;-7.73,6.03,)| Show InChI InChI=1S/C18H28N4O6S4/c1-9(23)19-13(15(25)26)7-31-17(29)21-11-3-5-12(6-4-11)22-18(30)32-8-14(16(27)28)20-10(2)24/h11-14H,3-8H2,1-2H3,(H,19,23)(H,20,24)(H,21,29)(H,22,30)(H,25,26)(H,27,28)/t11-,12-,13-,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.74E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50138806
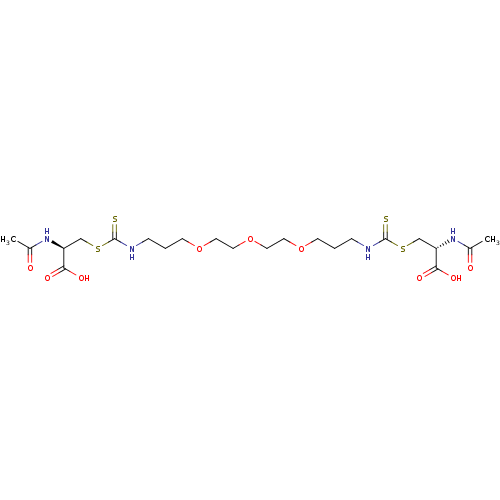
(CHEMBL3752941)Show SMILES CC(=O)N[C@@H](CSC(=S)NCCCOCCOCCOCCCNC(=S)SC[C@H](NC(C)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C22H38N4O9S4/c1-15(27)25-17(19(29)30)13-38-21(36)23-5-3-7-33-9-11-35-12-10-34-8-4-6-24-22(37)39-14-18(20(31)32)26-16(2)28/h17-18H,3-14H2,1-2H3,(H,23,36)(H,24,37)(H,25,27)(H,26,28)(H,29,30)(H,31,32)/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.26E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human LoVo/DX cells using Boc-Lys(Ac)-AMC as substrate assessed as release of AMC pre-incubated for 15 mins followed by substra... |
Bioorg Med Chem Lett 26: 667-71 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.045
BindingDB Entry DOI: 10.7270/Q2D220G2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data