 Found 106 hits with Last Name = 'zalaznick' and Initial = 'j'
Found 106 hits with Last Name = 'zalaznick' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hexokinase-4
(Homo sapiens (Human)) | BDBM50585812
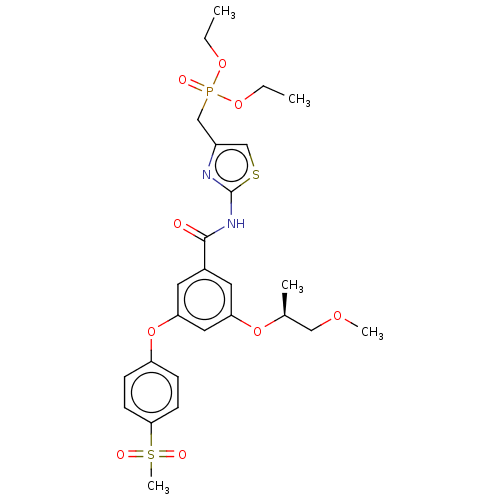
(CHEMBL5091943)Show SMILES CCOP(=O)(Cc1csc(NC(=O)c2cc(O[C@@H](C)COC)cc(Oc3ccc(cc3)S(C)(=O)=O)c2)n1)OCC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent labeled derivative from recombinant human hepatic glucokinase incubated for 30 mins in presence of 12 mM glucose by fluor... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50585813
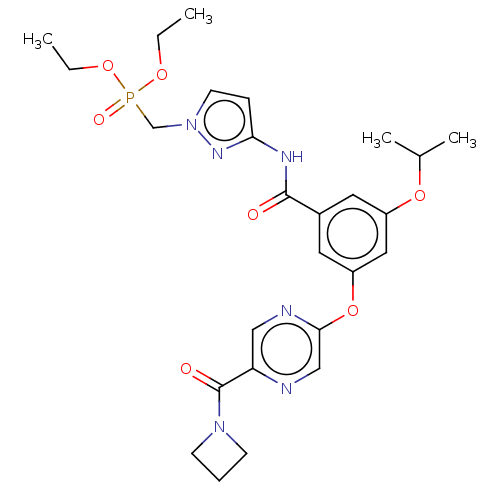
(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent labeled derivative from recombinant human hepatic glucokinase incubated for 30 mins in presence of 12 mM glucose by fluor... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50026240

(CHEMBL3338194)Show SMILES CS(=O)(=O)c1ccc(c(F)c1)-n1cc(Cl)c(OC2CCN(CC2)c2ncc(Cl)cn2)cc1=O Show InChI InChI=1S/C21H19Cl2FN4O4S/c1-33(30,31)15-2-3-18(17(24)8-15)28-12-16(23)19(9-20(28)29)32-14-4-6-27(7-5-14)21-25-10-13(22)11-26-21/h2-3,8-12,14H,4-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585829
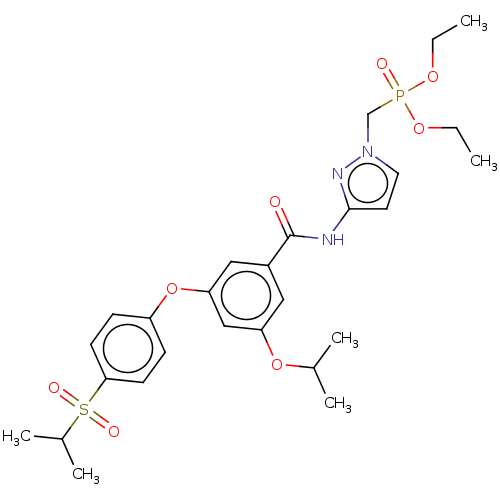
(CHEMBL5092846)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3ccc(cc3)S(=O)(=O)C(C)C)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2B6 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585830
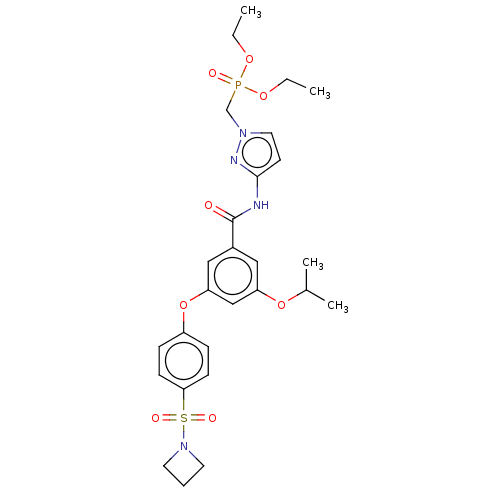
(CHEMBL5072442)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3ccc(cc3)S(=O)(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585823
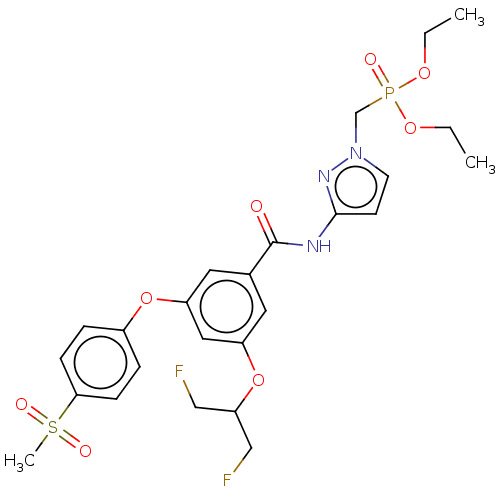
(CHEMBL5081517)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(CF)CF)cc(Oc3ccc(cc3)S(C)(=O)=O)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585812

(CHEMBL5091943)Show SMILES CCOP(=O)(Cc1csc(NC(=O)c2cc(O[C@@H](C)COC)cc(Oc3ccc(cc3)S(C)(=O)=O)c2)n1)OCC |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of P-gp transporter (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585826

(CHEMBL5085491)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(nc3)S(C)(=O)=O)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585824
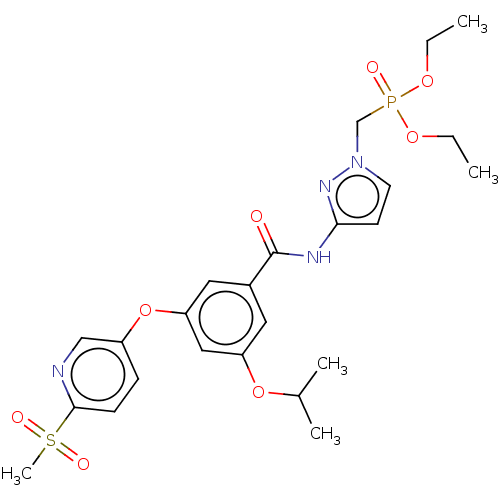
(CHEMBL5084870)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3ccc(nc3)S(C)(=O)=O)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585831
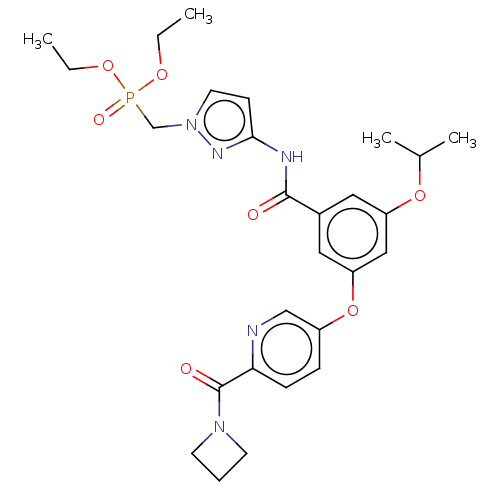
(CHEMBL5076377)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3ccc(nc3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585832
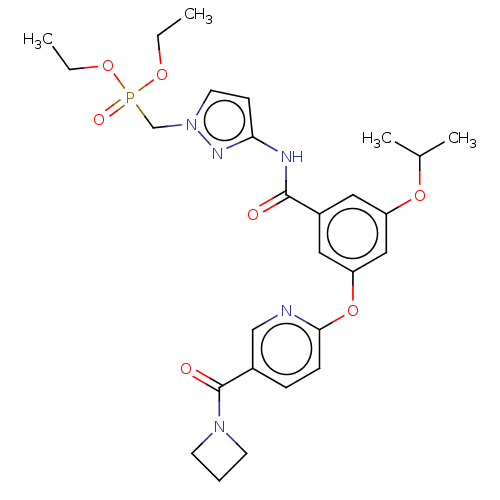
(CHEMBL5087489)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3ccc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585813

(CHEMBL5072532)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585833
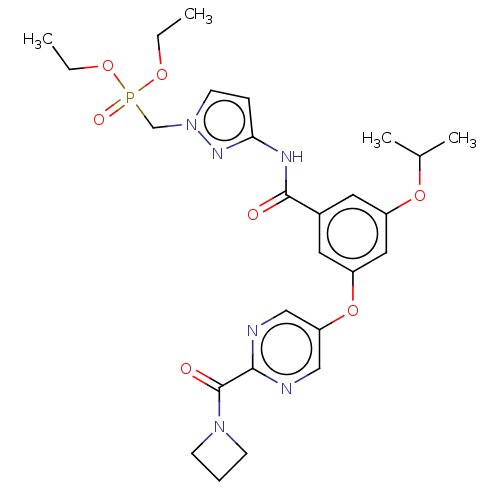
(CHEMBL5094606)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(nc3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585834

(CHEMBL5077438)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3ccc(nn3)C(=O)N3CCC3)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585835
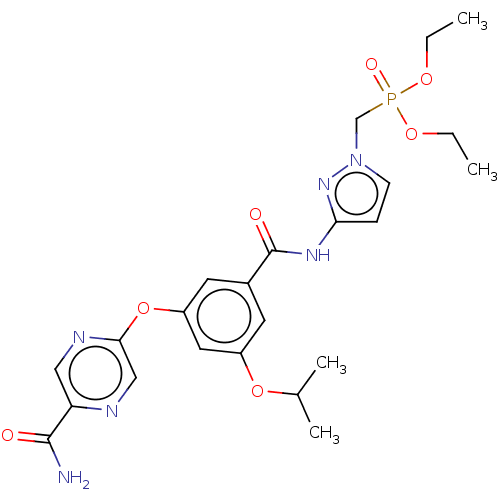
(CHEMBL5079866)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(N)=O)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585836
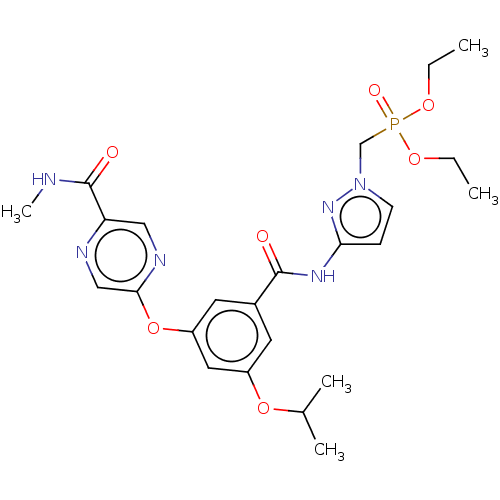
(CHEMBL5082691)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)NC)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585837

(CHEMBL5080166)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)C(=O)N(C)C)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585822
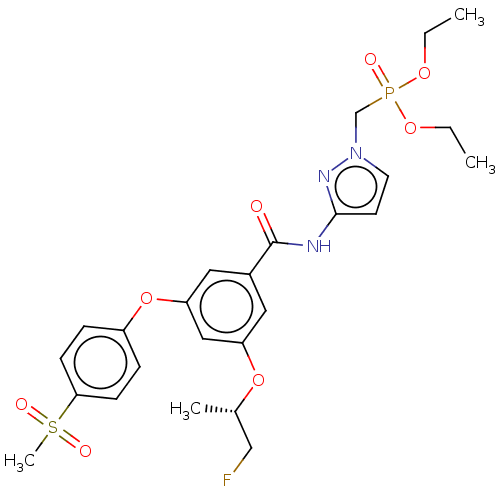
(CHEMBL5083369)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(O[C@@H](C)CF)cc(Oc3ccc(cc3)S(C)(=O)=O)c2)n1)OCC |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585821
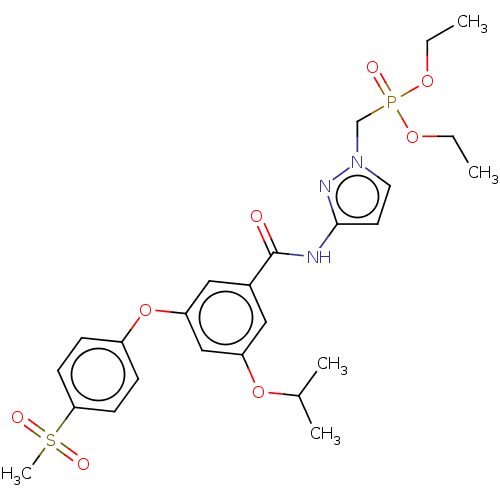
(CHEMBL5093978)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3ccc(cc3)S(C)(=O)=O)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585820
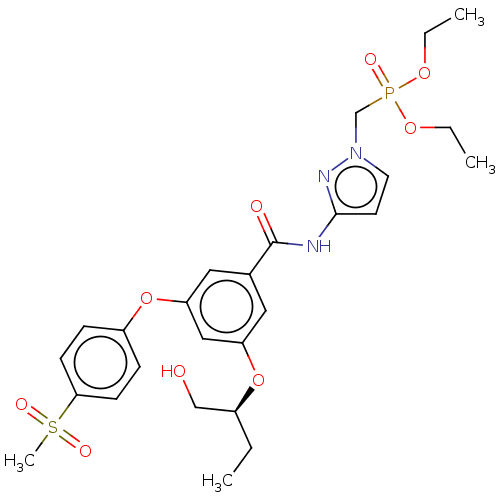
(CHEMBL5093208)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(O[C@@H](CC)CO)cc(Oc3ccc(cc3)S(C)(=O)=O)c2)n1)OCC |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585819
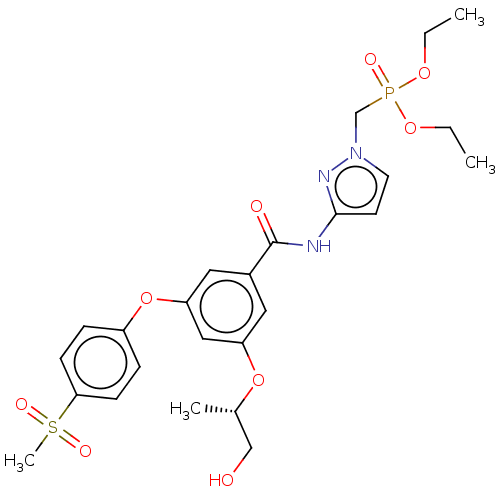
(CHEMBL5088988)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(O[C@@H](C)CO)cc(Oc3ccc(cc3)S(C)(=O)=O)c2)n1)OCC |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585818
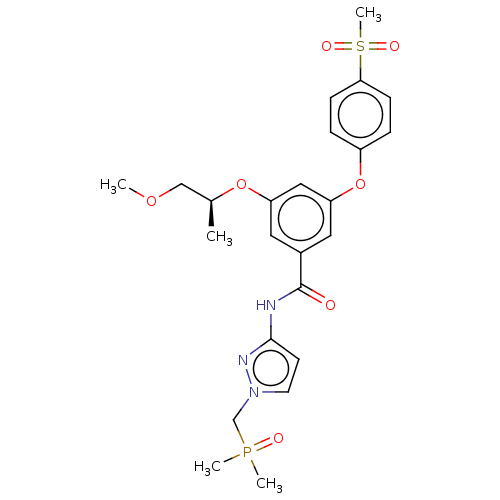
(CHEMBL5080889)Show SMILES COC[C@H](C)Oc1cc(Oc2ccc(cc2)S(C)(=O)=O)cc(c1)C(=O)Nc1ccn(CP(C)(C)=O)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585817

(CHEMBL5075882)Show SMILES CCO[P@](C)(=O)Cn1ccc(NC(=O)c2cc(O[C@@H](C)COC)cc(Oc3ccc(cc3)S(C)(=O)=O)c2)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585816

(CHEMBL5086985)Show SMILES CCOP(=O)(Cc1cnc(NC(=O)c2cc(O[C@@H](C)COC)cc(Oc3ccc(cc3)S(C)(=O)=O)c2)cn1)OCC |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585814
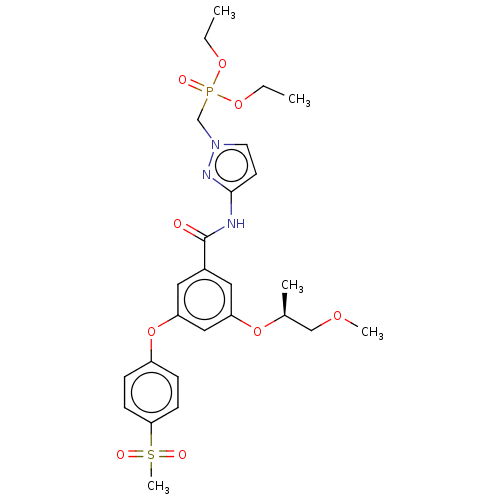
(CHEMBL5071861)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(O[C@@H](C)COC)cc(Oc3ccc(cc3)S(C)(=O)=O)c2)n1)OCC |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585827
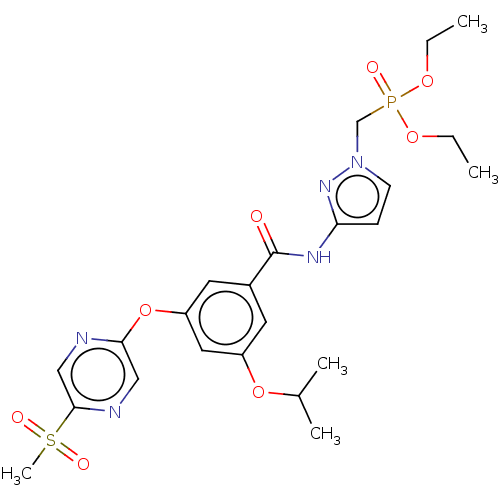
(CHEMBL5088522)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3cnc(cn3)S(C)(=O)=O)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50585825
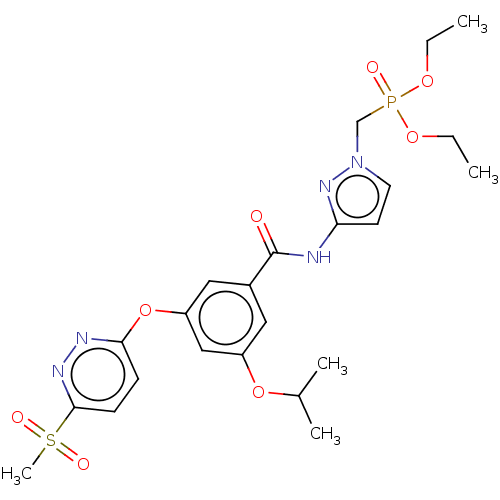
(CHEMBL5086686)Show SMILES CCOP(=O)(Cn1ccc(NC(=O)c2cc(OC(C)C)cc(Oc3ccc(nn3)S(C)(=O)=O)c2)n1)OCC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG by patch clamp method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02110
BindingDB Entry DOI: 10.7270/Q2057KV7 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026067
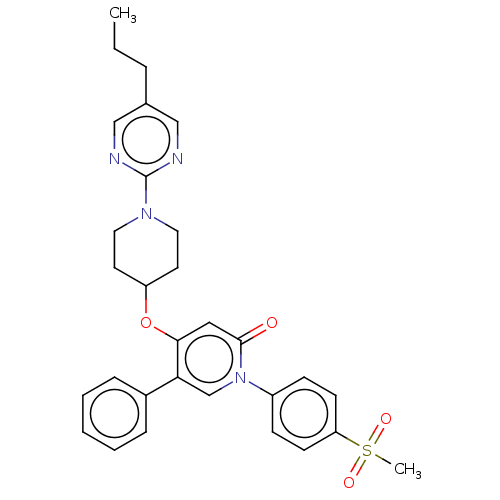
(CHEMBL3338186)Show SMILES CCCc1cnc(nc1)N1CCC(CC1)Oc1cc(=O)n(cc1-c1ccccc1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C30H32N4O4S/c1-3-7-22-19-31-30(32-20-22)33-16-14-25(15-17-33)38-28-18-29(35)34(21-27(28)23-8-5-4-6-9-23)24-10-12-26(13-11-24)39(2,36)37/h4-6,8-13,18-21,25H,3,7,14-17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026068
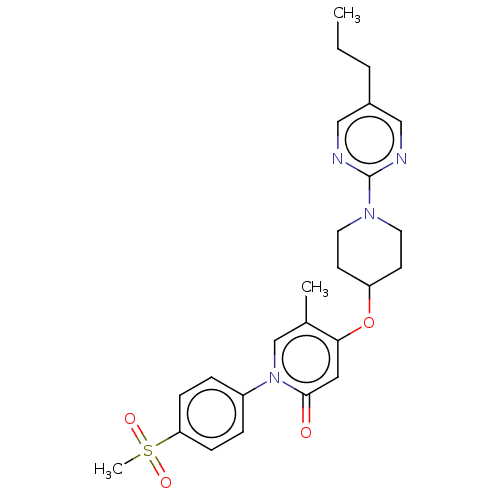
(CHEMBL3338185)Show SMILES CCCc1cnc(nc1)N1CCC(CC1)Oc1cc(=O)n(cc1C)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C25H30N4O4S/c1-4-5-19-15-26-25(27-16-19)28-12-10-21(11-13-28)33-23-14-24(30)29(17-18(23)2)20-6-8-22(9-7-20)34(3,31)32/h6-9,14-17,21H,4-5,10-13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026069
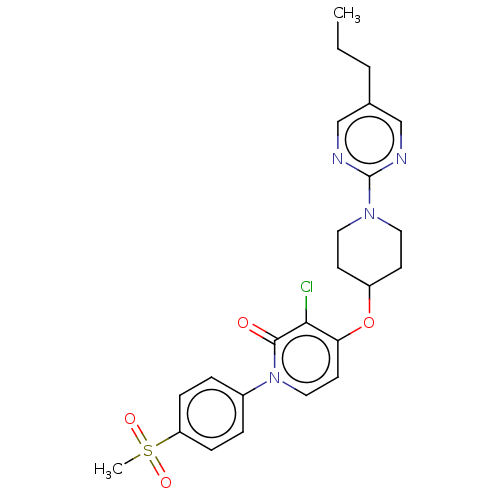
(CHEMBL3338184)Show SMILES CCCc1cnc(nc1)N1CCC(CC1)Oc1ccn(-c2ccc(cc2)S(C)(=O)=O)c(=O)c1Cl Show InChI InChI=1S/C24H27ClN4O4S/c1-3-4-17-15-26-24(27-16-17)28-12-9-19(10-13-28)33-21-11-14-29(23(30)22(21)25)18-5-7-20(8-6-18)34(2,31)32/h5-8,11,14-16,19H,3-4,9-10,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026070

(CHEMBL3338183)Show SMILES CCCc1cnc(nc1)N1CCC(CC1)Oc1cc(C)n(-c2ccc(cc2)S(C)(=O)=O)c(=O)c1 Show InChI InChI=1S/C25H30N4O4S/c1-4-5-19-16-26-25(27-17-19)28-12-10-21(11-13-28)33-22-14-18(2)29(24(30)15-22)20-6-8-23(9-7-20)34(3,31)32/h6-9,14-17,21H,4-5,10-13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026071
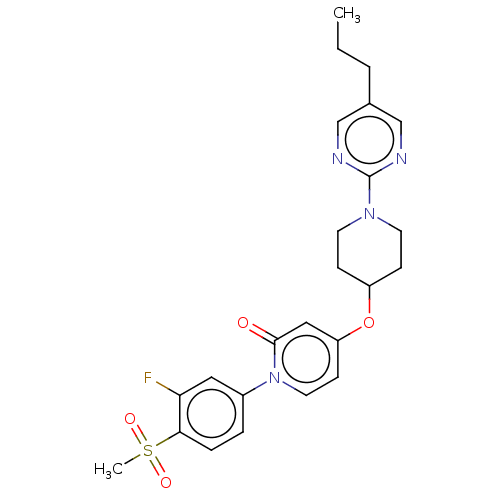
(CHEMBL3338182)Show SMILES CCCc1cnc(nc1)N1CCC(CC1)Oc1ccn(-c2ccc(c(F)c2)S(C)(=O)=O)c(=O)c1 Show InChI InChI=1S/C24H27FN4O4S/c1-3-4-17-15-26-24(27-16-17)28-10-7-19(8-11-28)33-20-9-12-29(23(30)14-20)18-5-6-22(21(25)13-18)34(2,31)32/h5-6,9,12-16,19H,3-4,7-8,10-11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026072

(CHEMBL3338181)Show SMILES CCCc1cnc(nc1)N1CCC(CC1)Oc1ccn(-c2ccc(cc2F)S(C)(=O)=O)c(=O)c1 Show InChI InChI=1S/C24H27FN4O4S/c1-3-4-17-15-26-24(27-16-17)28-10-7-18(8-11-28)33-19-9-12-29(23(30)13-19)22-6-5-20(14-21(22)25)34(2,31)32/h5-6,9,12-16,18H,3-4,7-8,10-11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026073

(CHEMBL3338180)Show SMILES CCCc1cnc(nc1)N1CCC(CC1)Oc1ccn(-c2ccc(cc2)-n2ccnc2)c(=O)c1 Show InChI InChI=1S/C26H28N6O2/c1-2-3-20-17-28-26(29-18-20)30-12-8-23(9-13-30)34-24-10-14-32(25(33)16-24)22-6-4-21(5-7-22)31-15-11-27-19-31/h4-7,10-11,14-19,23H,2-3,8-9,12-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 95 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026074
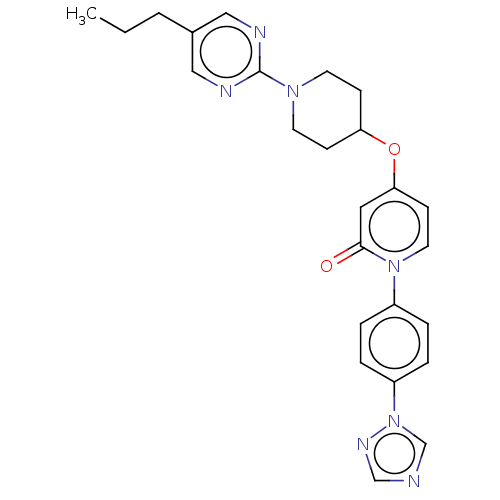
(CHEMBL3338179)Show SMILES CCCc1cnc(nc1)N1CCC(CC1)Oc1ccn(-c2ccc(cc2)-n2cncn2)c(=O)c1 Show InChI InChI=1S/C25H27N7O2/c1-2-3-19-15-27-25(28-16-19)30-11-8-22(9-12-30)34-23-10-13-31(24(33)14-23)20-4-6-21(7-5-20)32-18-26-17-29-32/h4-7,10,13-18,22H,2-3,8-9,11-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026075
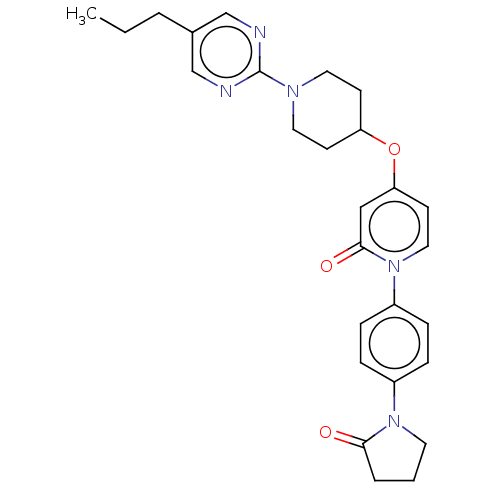
(CHEMBL3338178)Show SMILES CCCc1cnc(nc1)N1CCC(CC1)Oc1ccn(-c2ccc(cc2)N2CCCC2=O)c(=O)c1 Show InChI InChI=1S/C27H31N5O3/c1-2-4-20-18-28-27(29-19-20)30-14-10-23(11-15-30)35-24-12-16-32(26(34)17-24)22-8-6-21(7-9-22)31-13-3-5-25(31)33/h6-9,12,16-19,23H,2-5,10-11,13-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026076

(CHEMBL3338177)Show SMILES CCCc1cnc(nc1)N1CCC(CC1)Oc1ccn(-c2ccc(NC(=O)C(C)(C)C)cc2)c(=O)c1 Show InChI InChI=1S/C28H35N5O3/c1-5-6-20-18-29-27(30-19-20)32-14-11-23(12-15-32)36-24-13-16-33(25(34)17-24)22-9-7-21(8-10-22)31-26(35)28(2,3)4/h7-10,13,16-19,23H,5-6,11-12,14-15H2,1-4H3,(H,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026077

(CHEMBL3338176)Show SMILES CCCc1cnc(nc1)N1CCC(CC1)Oc1ccn(-c2ccc(cc2)C#N)c(=O)c1 Show InChI InChI=1S/C24H25N5O2/c1-2-3-19-16-26-24(27-17-19)28-11-8-21(9-12-28)31-22-10-13-29(23(30)14-22)20-6-4-18(15-25)5-7-20/h4-7,10,13-14,16-17,21H,2-3,8-9,11-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026175

(CHEMBL3338175)Show SMILES CCCc1cnc(nc1)N1CCC(CC1)Oc1ccn(-c2ccc(cc2)[S+](C)[O-])c(=O)c1 Show InChI InChI=1S/C24H28N4O3S/c1-3-4-18-16-25-24(26-17-18)27-12-9-20(10-13-27)31-21-11-14-28(23(29)15-21)19-5-7-22(8-6-19)32(2)30/h5-8,11,14-17,20H,3-4,9-10,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026176
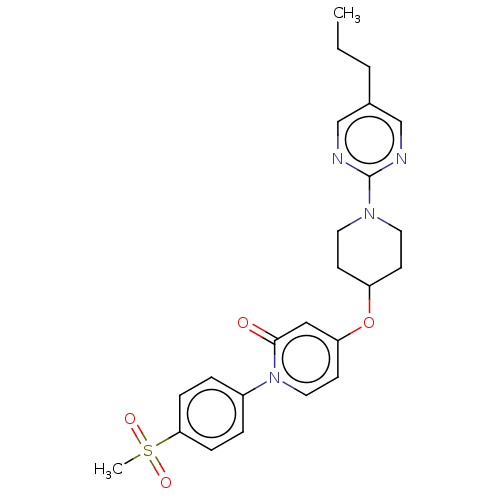
(CHEMBL3338174)Show SMILES CCCc1cnc(nc1)N1CCC(CC1)Oc1ccn(-c2ccc(cc2)S(C)(=O)=O)c(=O)c1 Show InChI InChI=1S/C24H28N4O4S/c1-3-4-18-16-25-24(26-17-18)27-12-9-20(10-13-27)32-21-11-14-28(23(29)15-21)19-5-7-22(8-6-19)33(2,30)31/h5-8,11,14-17,20H,3-4,9-10,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026177

(CHEMBL3338173)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1ccc(OC2CCN(CC2)c2ncc(cn2)C2CC2)cc1=O Show InChI InChI=1S/C24H26N4O4S/c1-33(30,31)22-6-4-19(5-7-22)28-13-10-21(14-23(28)29)32-20-8-11-27(12-9-20)24-25-15-18(16-26-24)17-2-3-17/h4-7,10,13-17,20H,2-3,8-9,11-12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50026178

(CHEMBL3338172)Show SMILES COc1cnc(nc1)N1CCC(CC1)Oc1ccn(-c2ccc(cc2)S(C)(=O)=O)c(=O)c1 Show InChI InChI=1S/C22H24N4O5S/c1-30-19-14-23-22(24-15-19)25-10-7-17(8-11-25)31-18-9-12-26(21(27)13-18)16-3-5-20(6-4-16)32(2,28)29/h3-6,9,12-15,17H,7-8,10-11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
Departments of Discovery Chemistry, Metabolic Diseases, Lead Evaluation, Computer-Assisted Drug Design, Discovery Toxicology, Exploratory Clinical and Translational Research, and Pharmaceutical Candi
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 |
J Med Chem 57: 7499-508 (2014)
Article DOI: 10.1021/jm501175v
BindingDB Entry DOI: 10.7270/Q2K35W7Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data