

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Collagenase 3 (Homo sapiens (Human)) | BDBM50084286 (2-(4'-Cyano-biphenyl-4-sulfonylamino)-N-hydroxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of human recombinant matrix metalloprotease-13 (MMP-13) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50084286 (2-(4'-Cyano-biphenyl-4-sulfonylamino)-N-hydroxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-2 (MMP-2) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM8485 ((2R)-N-hydroxy-3-methyl-2-[(4-phenoxybenzene)sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-2 (MMP-2) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM12076 ((2R)-2-{[4-(4-bromophenyl)benzene]sulfonamido}-N-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-2 (MMP-2) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50084291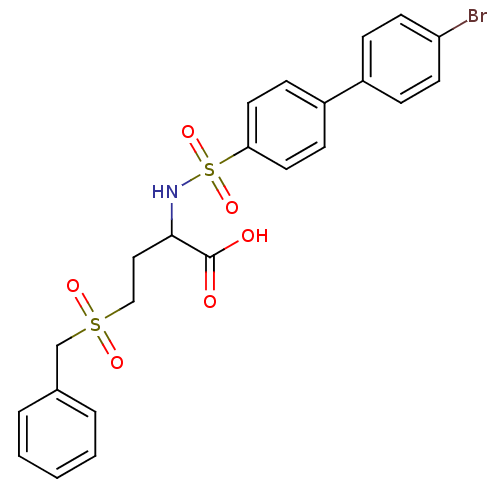 (2-(4'-Bromo-biphenyl-4-sulfonylamino)-4-phenylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-2 (MMP-2) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM12076 ((2R)-2-{[4-(4-bromophenyl)benzene]sulfonamido}-N-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of human recombinant matrix metalloprotease-13 (MMP-13) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50084283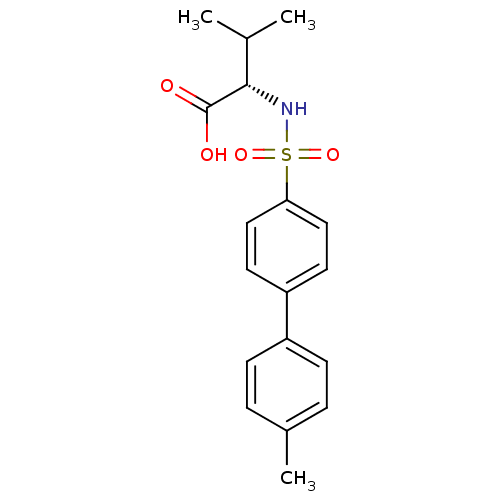 ((S)-3-methyl-2-(4'-methyl-biphenyl-4-sulfonylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-2 (MMP-2) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50011204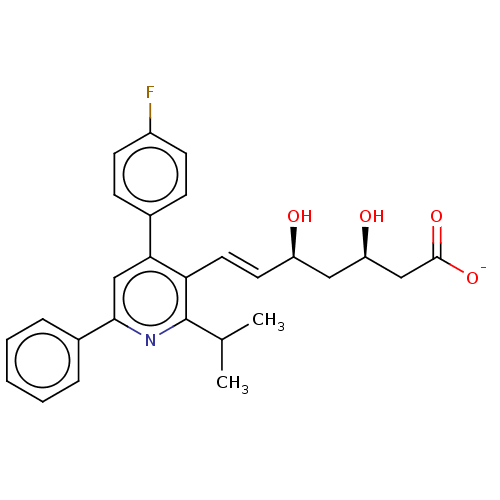 (CHEMBL2368094 | Sodium; 7-[4-(4-fluoro-phenyl)-2-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM8485 ((2R)-N-hydroxy-3-methyl-2-[(4-phenoxybenzene)sulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of human recombinant matrix metalloprotease-13 (MMP-13) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50084283 ((S)-3-methyl-2-(4'-methyl-biphenyl-4-sulfonylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of recombinant human matrix metalloprotease-3 (MMP-3) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM8485 ((2R)-N-hydroxy-3-methyl-2-[(4-phenoxybenzene)sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-9 (MMP-9) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50084289 ((S)-2-(4'-methoxy-biphenyl-4-sulfonylamino)-3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-2 (MMP-2) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50084297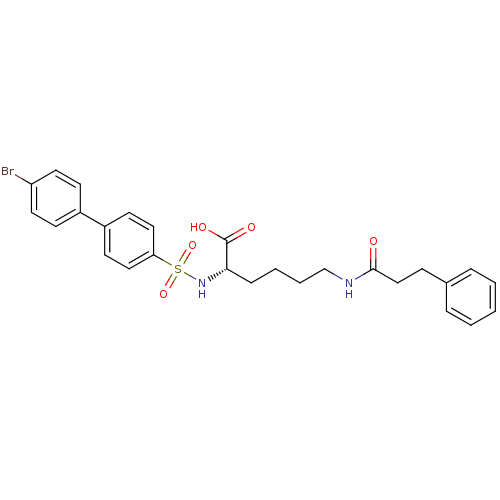 (2-(4'-Bromo-biphenyl-4-sulfonylamino)-6-(3-phenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of human recombinant matrix metalloprotease-13 (MMP-13) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50084297 (2-(4'-Bromo-biphenyl-4-sulfonylamino)-6-(3-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-2 (MMP-2) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM12075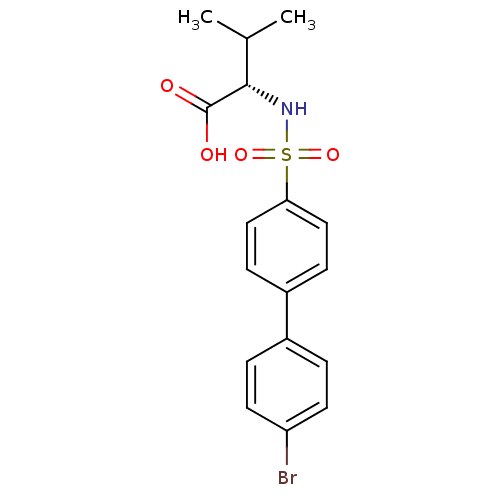 ((2S)-2-{[4-(4-bromophenyl)benzene]sulfonamido}-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-2 (MMP-2) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50289025 (CHEMBL164479 | N-(2,6-Diisopropyl-phenyl)-malonami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in intestinal microsomes isolated from cholesterol fed rabbits (IAI)... | Bioorg Med Chem Lett 6: 713-718 (1996) Article DOI: 10.1016/0960-894X(96)00098-4 BindingDB Entry DOI: 10.7270/Q2DV1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50084304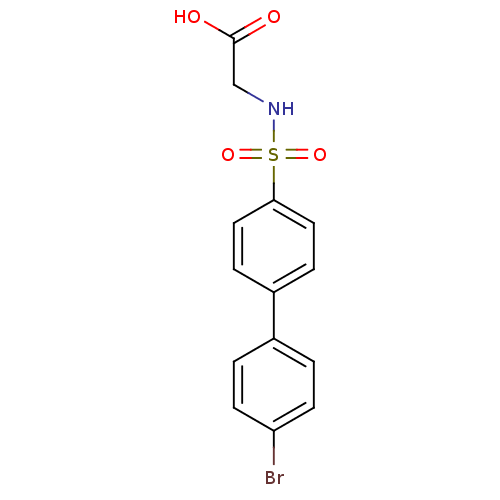 ((4'-Bromo-biphenyl-4-sulfonylamino)-acetic acid | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-2 (MMP-2) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50289051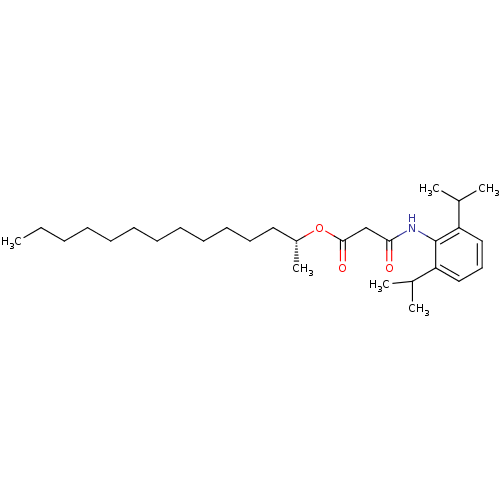 (CHEMBL160103 | N-(2,6-Diisopropyl-phenyl)-malonami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in intestinal microsomes isolated from cholesterol fed rabbits (IAI)... | Bioorg Med Chem Lett 6: 713-718 (1996) Article DOI: 10.1016/0960-894X(96)00098-4 BindingDB Entry DOI: 10.7270/Q2DV1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM8485 ((2R)-N-hydroxy-3-methyl-2-[(4-phenoxybenzene)sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of recombinant human matrix metalloprotease-3 (MMP-3) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50084281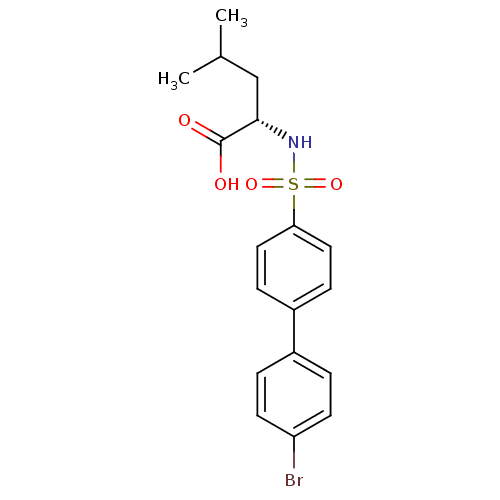 (2-(4'-Bromo-biphenyl-4-sulfonylamino)-4-methyl-pen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of human recombinant matrix metalloprotease-13 (MMP-13) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50084286 (2-(4'-Cyano-biphenyl-4-sulfonylamino)-N-hydroxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of recombinant human matrix metalloprotease-3 (MMP-3) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50084295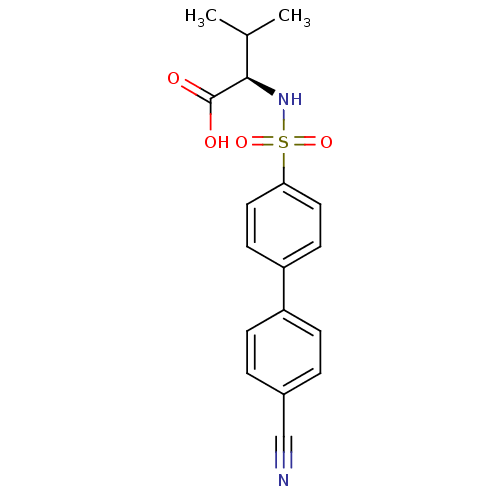 (2-(4'-Cyano-biphenyl-4-sulfonylamino)-3-methyl-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of recombinant human matrix metalloprotease-3 (MMP-3) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051461 (CHEMBL264861 | N-(2,6-Diisopropyl-phenyl)-2-(2-dod...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50084290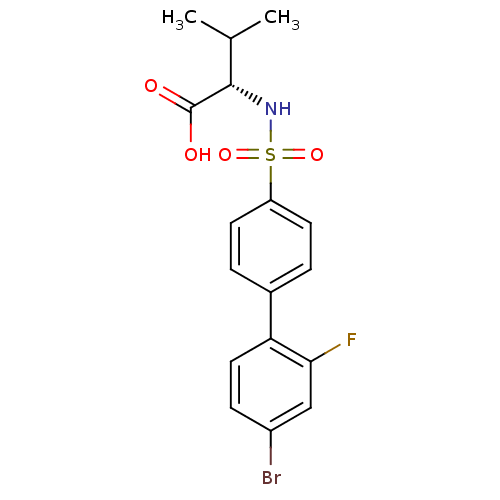 ((S)-2-(4'-bromo-2'-fluoro-biphenyl-4-sulfonylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-2 (MMP-2) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM12076 ((2R)-2-{[4-(4-bromophenyl)benzene]sulfonamido}-N-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of recombinant human matrix metalloprotease-3 (MMP-3) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM12074 ((2R)-2-{[4-(4-bromophenyl)benzene]sulfonamido}-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-2 (MMP-2) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50084287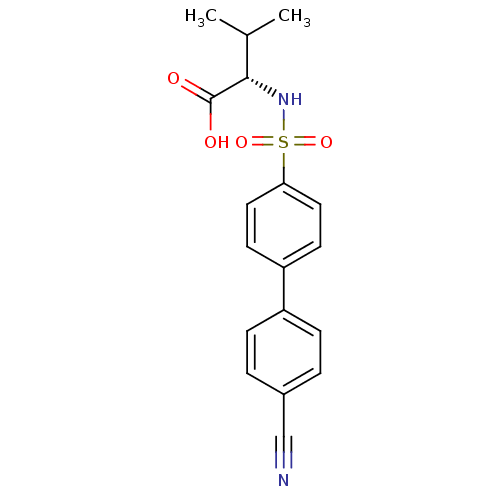 ((S)-2-(4'-cyano-biphenyl-4-sulfonylamino)-3-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of recombinant human matrix metalloprotease-3 (MMP-3) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50084289 ((S)-2-(4'-methoxy-biphenyl-4-sulfonylamino)-3-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of human recombinant matrix metalloprotease-13 (MMP-13) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50051466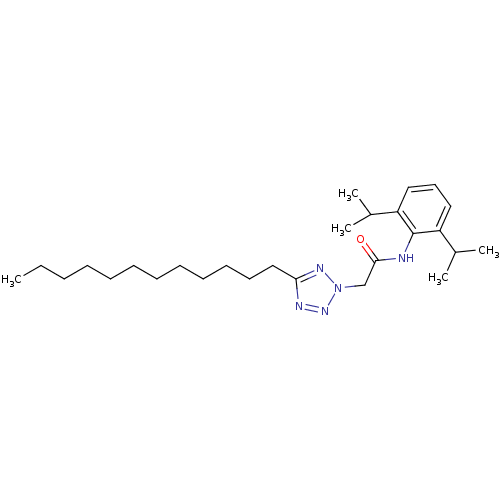 (CHEMBL175982 | N-(2,6-Diisopropyl-phenyl)-2-(5-dod...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against acyl coenzyme A:cholesterol acyltransferase 1 of liver microsomes isolated from cholesterol-fed rats. | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50289024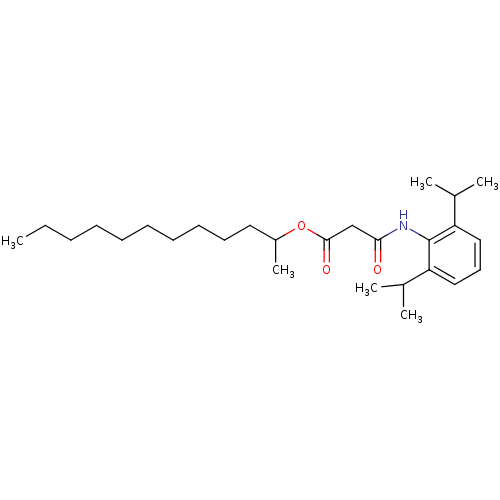 (CHEMBL422709 | N-(2,6-Diisopropyl-phenyl)-malonami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Acyl coenzyme A:cholesterol acyltransferase in intestinal microsomes isolated from cholesterol fed rabbits (IAI)... | Bioorg Med Chem Lett 6: 713-718 (1996) Article DOI: 10.1016/0960-894X(96)00098-4 BindingDB Entry DOI: 10.7270/Q2DV1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50006407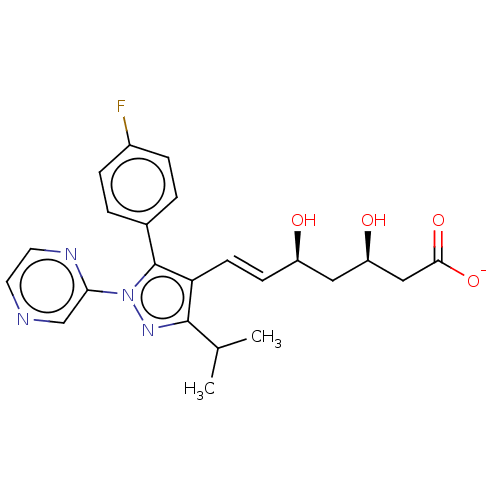 (CHEMBL2367472 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50084290 ((S)-2-(4'-bromo-2'-fluoro-biphenyl-4-sulfonylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of human recombinant matrix metalloprotease-13 (MMP-13) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50006407 (CHEMBL2367472 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested in vitro for the inhibition of HMG-CoA reductase from partially purified microsomal preparations. | J Med Chem 35: 2095-103 (1992) BindingDB Entry DOI: 10.7270/Q2HH6KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50084281 (2-(4'-Bromo-biphenyl-4-sulfonylamino)-4-methyl-pen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of recombinant human matrix metalloprotease-3 (MMP-3) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50084291 (2-(4'-Bromo-biphenyl-4-sulfonylamino)-4-phenylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of recombinant human matrix metalloprotease-3 (MMP-3) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50006407 (CHEMBL2367472 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested in vitro for the inhibition of HMG-CoA reductase from partially purified microsomal preparations. | J Med Chem 35: 2095-103 (1992) BindingDB Entry DOI: 10.7270/Q2HH6KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM12075 ((2S)-2-{[4-(4-bromophenyl)benzene]sulfonamido}-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of recombinant human matrix metalloprotease-3 (MMP-3) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50069883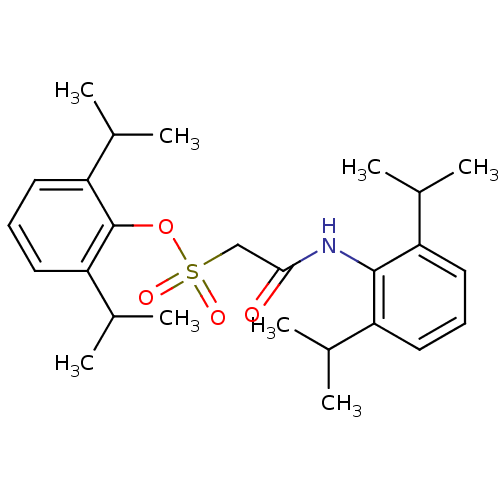 ((2,6-Diisopropyl-phenylcarbamoyl)-methanesulfonic ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of ACAT by incubation with [1-14C]-oleolyl-CoA and intestinal microsomes isolated from cholesterol-fed rabbits. | Bioorg Med Chem Lett 8: 289-94 (1999) BindingDB Entry DOI: 10.7270/Q2DN446R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50069886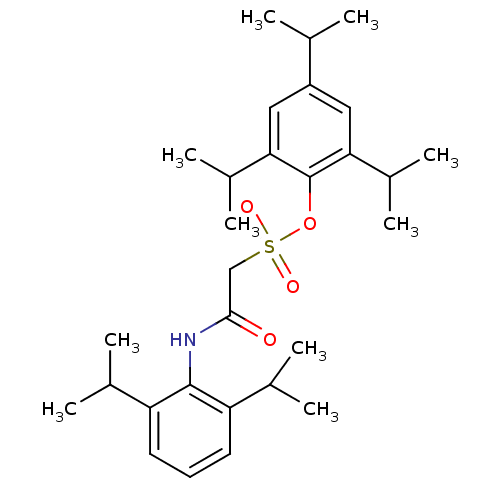 ((2,6-Diisopropyl-phenylcarbamoyl)-methanesulfonic ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibition of ACAT by incubation with [1-14C]-oleolyl-CoA and intestinal microsomes isolated from cholesterol-fed rabbits. | Bioorg Med Chem Lett 8: 289-94 (1999) BindingDB Entry DOI: 10.7270/Q2DN446R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50006409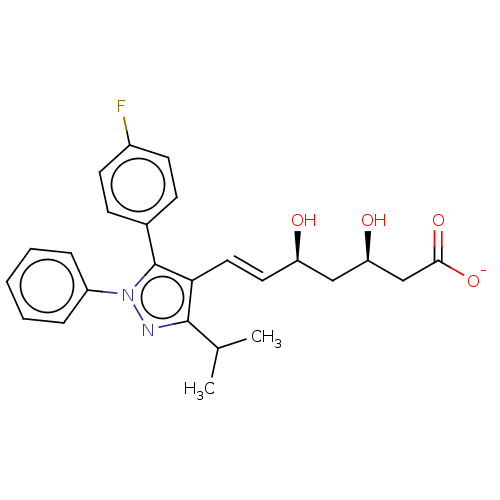 (CHEMBL2367478 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Tested in vitro for the inhibition of HMG-CoA reductase from partially purified microsomal preparations. | J Med Chem 35: 2095-103 (1992) BindingDB Entry DOI: 10.7270/Q2HH6KN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50084295 (2-(4'-Cyano-biphenyl-4-sulfonylamino)-3-methyl-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-2 (MMP-2) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50084298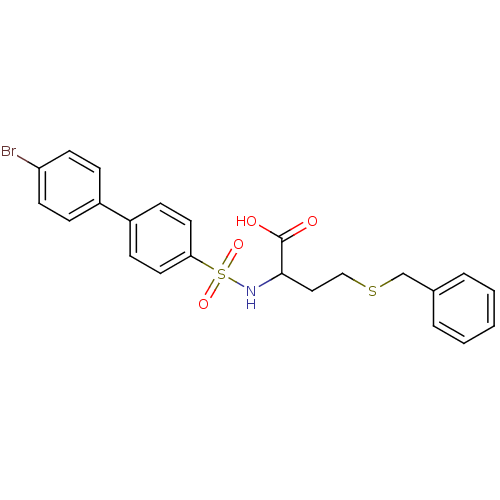 (4-Benzylsulfanyl-2-(4'-bromo-biphenyl-4-sulfonylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of matrix metalloprotease-2 (MMP-2) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50084300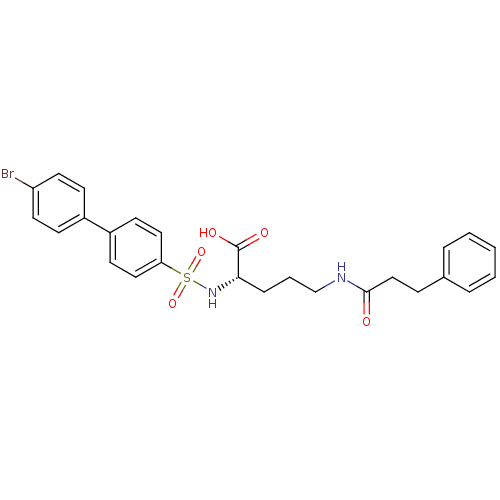 (2-(4'-Bromo-biphenyl-4-sulfonylamino)-5-(3-phenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of human recombinant matrix metalloprotease-13 (MMP-13) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50006409 (CHEMBL2367478 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50011213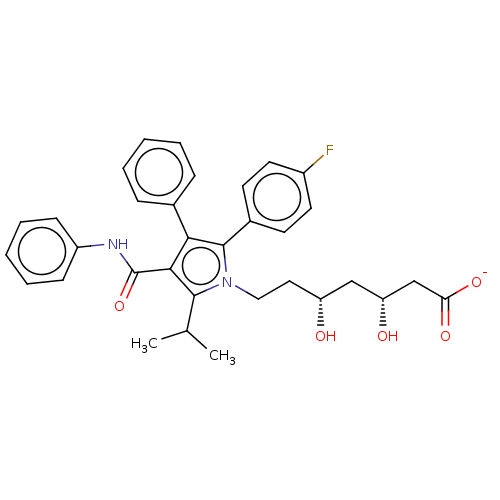 (CHEMBL3349878 | Sodium; 7-[2-(4-fluoro-phenyl)-5-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50011211 (CHEMBL2368089 | Sodium; 7-[4-(4-fluoro-phenyl)-2-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50084302 ((S)-2-(4'-formyl-biphenyl-4-sulfonylamino)-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of recombinant human matrix metalloprotease-3 (MMP-3) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50063243 (CHEMBL161499 | N-(2,6-Diisopropyl-phenyl)-2-pyridi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro Acyl coenzyme A:cholesterol acyltransferase inhibition in hepatic microsomes isolated from cholesterol-fed rats | J Med Chem 41: 682-90 (1998) Article DOI: 10.1021/jm970560h BindingDB Entry DOI: 10.7270/Q28051R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM12074 ((2R)-2-{[4-(4-bromophenyl)benzene]sulfonamido}-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro inhibition of recombinant human matrix metalloprotease-3 (MMP-3) | J Med Chem 43: 156-66 (2000) BindingDB Entry DOI: 10.7270/Q2T154BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50051460 (2-(2-Dodecyl-2H-tetrazol-5-yl)-2-phenyl-N-(2,4,6-t...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase 1 from rabbit intestine | J Med Chem 39: 2354-66 (1996) Article DOI: 10.1021/jm960170f BindingDB Entry DOI: 10.7270/Q2W66JVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 719 total ) | Next | Last >> |