

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50040423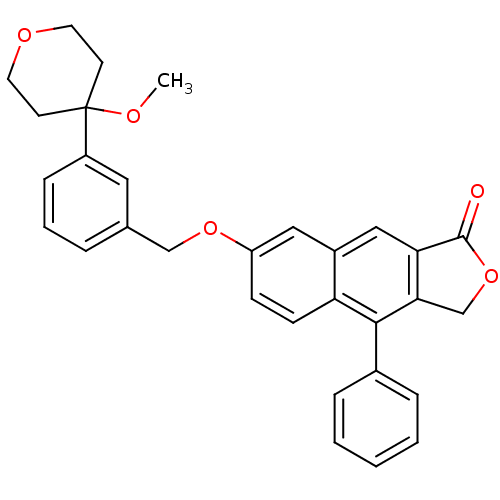 (7-(3-(4-methoxytetrahydro-2H-pyran-4-yl)benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053534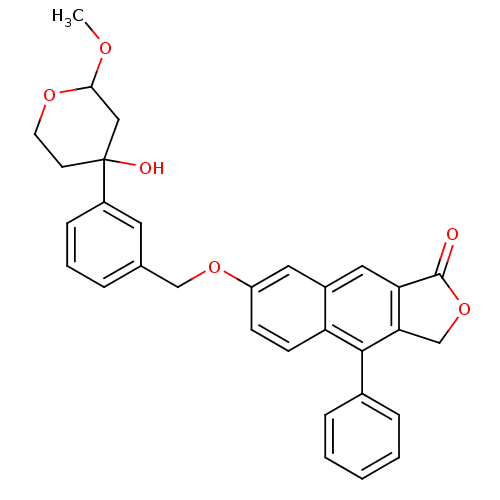 (7-[3-(4-Hydroxy-2-methoxy-tetrahydro-pyran-4-yl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053537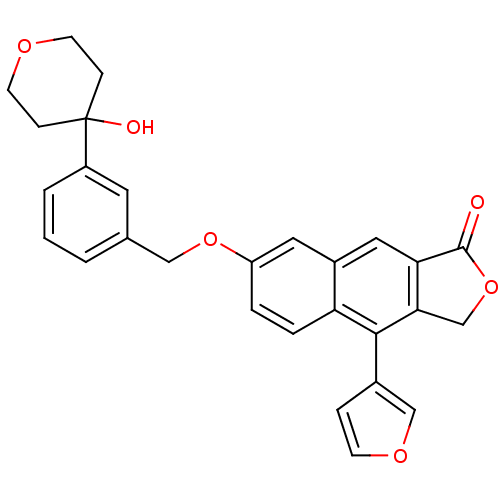 (4-Furan-3-yl-7-[3-(4-hydroxy-tetrahydro-pyran-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053564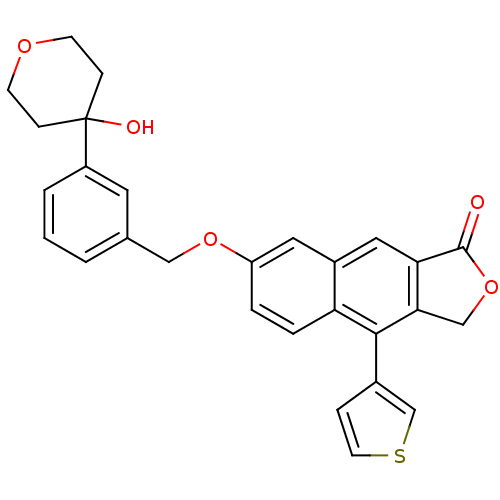 (7-[3-(4-Hydroxy-tetrahydro-pyran-4-yl)-benzyloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053554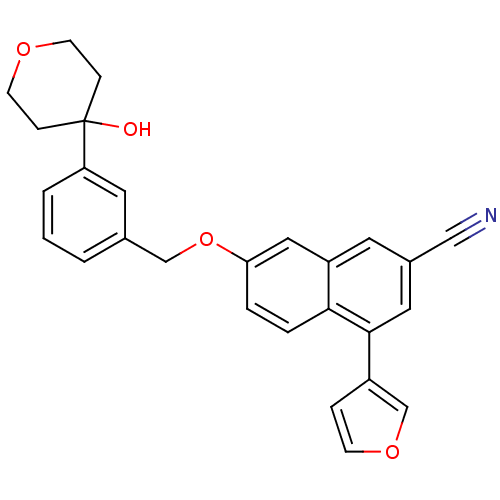 (4-Furan-3-yl-7-[3-(4-hydroxy-tetrahydro-pyran-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053529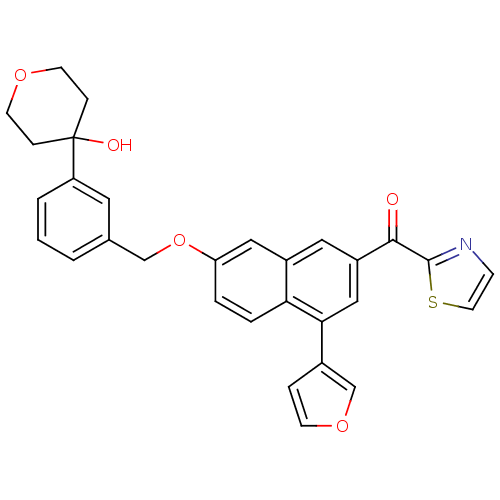 (CHEMBL127779 | {4-Furan-3-yl-7-[3-(4-hydroxy-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053566 (4-{3-[7-(4,5-Dihydro-thiazol-2-yl)-5-furan-3-yl-na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053549 (CHEMBL338461 | {4-Furan-3-yl-7-[3-(4-hydroxy-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053543 (7-[3-(4-Hydroxy-tetrahydro-pyran-4-yl)-benzyloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053539 (4-{3-[7-(4,5-Dihydro-oxazol-2-yl)-5-furan-3-yl-nap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053538 (7-[3-(4-Hydroxy-tetrahydro-pyran-4-yl)-benzyloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053518 (4-{3-[5-Furan-3-yl-7-(3-methyl-1H-tetrazol-5-yl)-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053542 (7-[3-(4-Hydroxy-tetrahydro-pyran-4-yl)-benzyloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053521 (7-[3-(4-Hydroxy-tetrahydro-pyran-4-yl)-benzyloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053527 (4-{3-[7-(3-Methyl-1H-tetrazol-5-yl)-5-phenyl-napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053523 (CHEMBL340737 | {4-Furan-3-yl-7-[3-(4-hydroxy-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053522 (1-{7-[3-(4-Hydroxy-tetrahydro-pyran-4-yl)-benzylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053557 (4-[3-(5-Furan-3-yl-7-thiazol-2-ylmethyl-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053540 (4-Furan-3-yl-7-[3-(3-hydroxy-6,8-dioxa-bicyclo[3.2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053540 (4-Furan-3-yl-7-[3-(3-hydroxy-6,8-dioxa-bicyclo[3.2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053551 (4-(4-Fluoro-phenyl)-7-[3-(4-hydroxy-tetrahydro-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053553 (4-[3-(7-Methoxymethyl-5-phenyl-naphthalen-2-yloxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053525 (7-[3-(4-Hydroxy-2,6-dimethyl-tetrahydro-pyran-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053565 (4-{3-[7-(4,4-Dimethyl-4,5-dihydro-oxazol-2-yl)-5-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053548 (4-Furan-3-yl-7-[3-(3-hydroxy-6,8-dioxa-bicyclo[3.2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053519 (7-[3-(3-Hydroxy-6,8-dioxa-bicyclo[3.2.1]oct-3-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053555 (4-[3-(7-Hydroxymethyl-5-phenyl-naphthalen-2-yloxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053558 (4-Furan-2-yl-7-[3-(4-hydroxy-tetrahydro-pyran-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50080081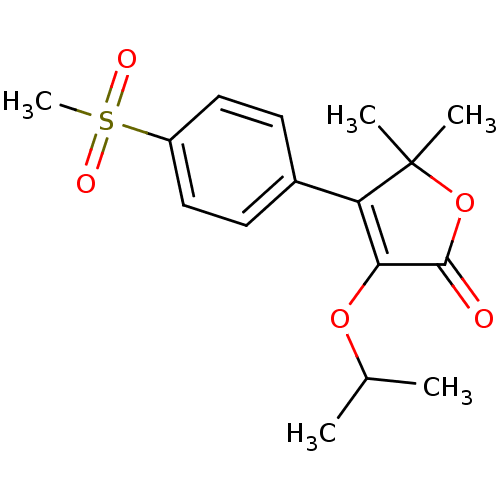 (3-Isopropoxy-4-(4-methanesulfonyl-phenyl)-5,5-dime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibitory potency of the compound was determined against Prostaglandin G/H synthase 2 in human whole blood assay | Bioorg Med Chem Lett 12: 3317-20 (2002) BindingDB Entry DOI: 10.7270/Q2FQ9VZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053562 (7-[3-(3-Hydroxy-8-oxa-bicyclo[3.2.1]oct-3-yl)-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000829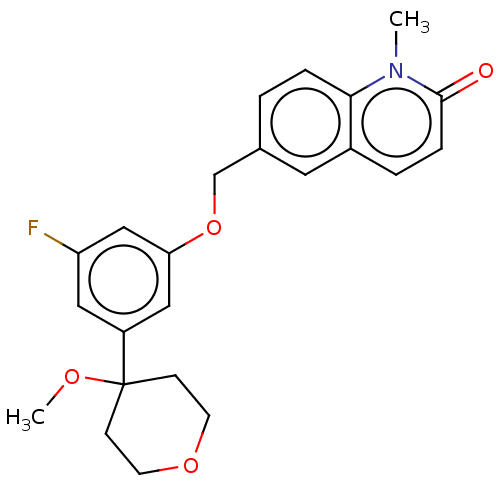 (6-((3-fluoro-5-(4-methoxytetrahydro-2H-pyran-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053520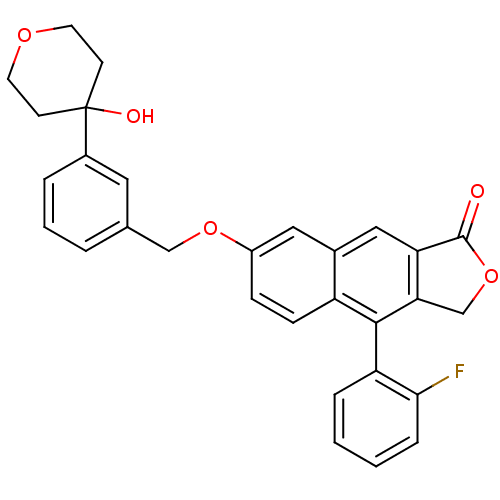 (4-(2-Fluoro-phenyl)-7-[3-(4-hydroxy-tetrahydro-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053561 (4-(2-Chloro-phenyl)-7-[3-(4-hydroxy-tetrahydro-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053531 (7-[3-(4-Hydroxy-2-oxo-tetrahydro-pyran-4-yl)-benzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053547 (7-[3-(3-Hydroxy-8-oxa-bicyclo[3.2.1]oct-6-en-3-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50120573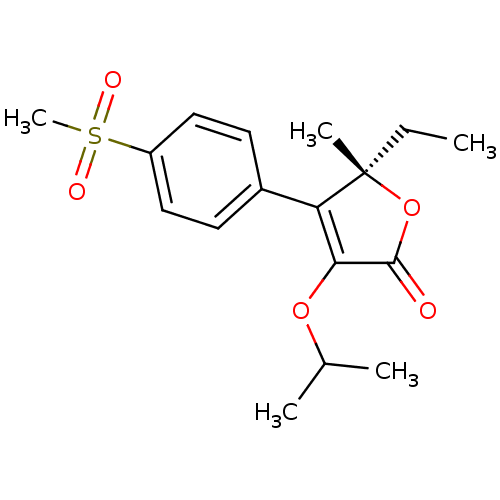 ((S)-5-Ethyl-3-isopropoxy-4-(4-methanesulfonyl-phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibitory potency of the compound was determined against Prostaglandin G/H synthase 2 in human whole blood assay | Bioorg Med Chem Lett 12: 3317-20 (2002) BindingDB Entry DOI: 10.7270/Q2FQ9VZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50120571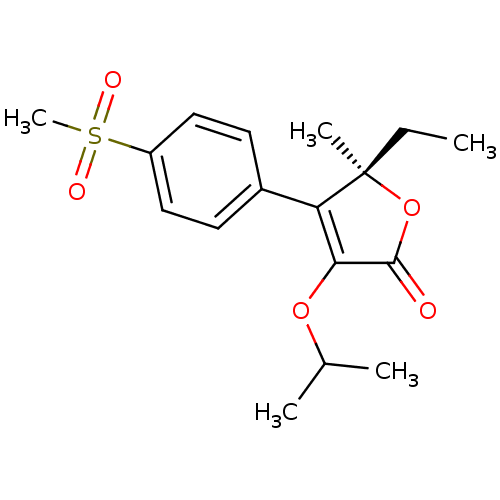 (5-Ethyl-3-isopropoxy-4-(4-methanesulfonyl-phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibitory potency of the compound was determined against Prostaglandin G/H synthase 2 in human whole blood assay | Bioorg Med Chem Lett 12: 3317-20 (2002) BindingDB Entry DOI: 10.7270/Q2FQ9VZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053526 (4-{3-[7-(1-Hydroxy-ethyl)-5-phenyl-naphthalen-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against COX- 2. | Bioorg Med Chem Lett 10: 2683-6 (2000) BindingDB Entry DOI: 10.7270/Q2GT5MFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053559 (4-[3-(7-Imidazol-1-ylmethyl-5-phenyl-naphthalen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053545 (7-[3-(4-Hydroxy-tetrahydro-pyran-4-yl)-benzyloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053541 (4-[3-(7-Ethyl-5-phenyl-naphthalen-2-yloxymethyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053533 (7-[3-(2,4-Dihydroxy-tetrahydro-pyran-4-yl)-benzylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50072064 (5-Chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibition of Prostaglandin G/H synthase 2 in human whole blood assay | Bioorg Med Chem Lett 11: 1059-62 (2001) BindingDB Entry DOI: 10.7270/Q25Q4VC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053560 (4-{3-[7-(1-Methoxy-ethyl)-5-phenyl-naphthalen-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053528 (7-[3-(4-Hydroxy-tetrahydro-pyran-4-yl)-benzyloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50120572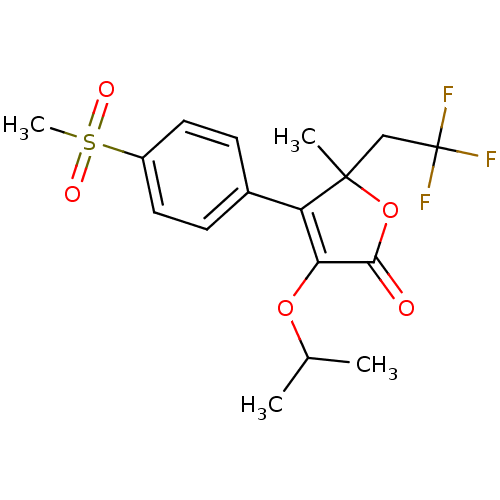 (3-Isopropoxy-4-(4-methanesulfonyl-phenyl)-5-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibitory potency of the compound was determined against Prostaglandin G/H synthase 2 in human whole blood assay | Bioorg Med Chem Lett 12: 3317-20 (2002) BindingDB Entry DOI: 10.7270/Q2FQ9VZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053524 (4-{3-[7-(1-Hydroxy-1-methyl-ethyl)-5-phenyl-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053536 (4-Butyl-7-[3-(4-hydroxy-tetrahydro-pyran-4-yl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50053530 (4-{3-[7-(4-Methyl-1H-tetrazol-5-yl)-5-phenyl-napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Potency to inhibit oxidation of arachidonic acid by recombinant human 5-lipoxygenase | J Med Chem 39: 3951-70 (1996) Article DOI: 10.1021/jm960301c BindingDB Entry DOI: 10.7270/Q2639QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 89 total ) | Next | Last >> |