

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at day 8 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025972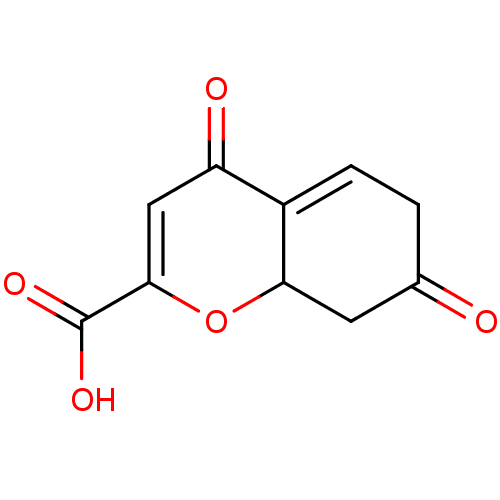 (7-Hydroxy-4-oxo-4a,8a-dihydro-4H-chromene-2-carbox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at day 7 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025978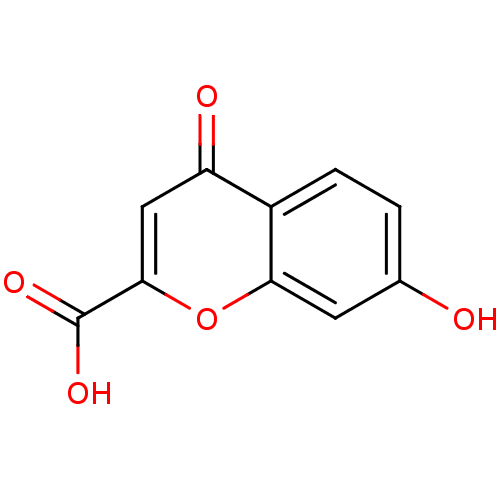 (7-Hydroxy-4-oxo-4H-chromene-2-carboxylic acid | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase (HPAR). | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50025978 (7-Hydroxy-4-oxo-4H-chromene-2-carboxylic acid | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against rat lens aldose reductase (RLAR). | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50025973 (7-Hydroxy-4-oxo-4H-chromene-2-carboxylic acid ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against rat lens aldose reductase (RLAR). | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50025980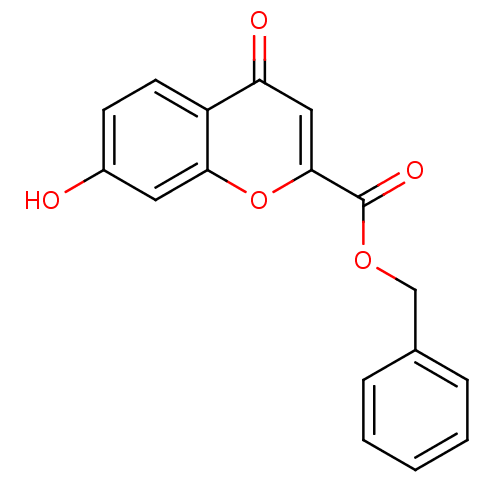 (7-Hydroxy-4-oxo-4H-chromene-2-carboxylic acid benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against rat lens aldose reductase (RLAR). | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025972 (7-Hydroxy-4-oxo-4a,8a-dihydro-4H-chromene-2-carbox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at day 8 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50025977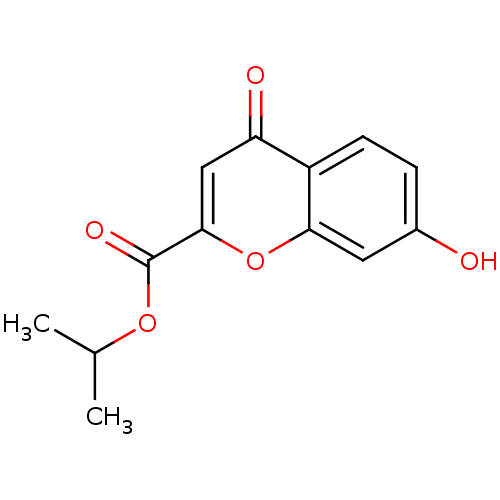 (7-Hydroxy-4-oxo-4H-chromene-2-carboxylic acid isop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against rat lens aldose reductase (RLAR). | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025979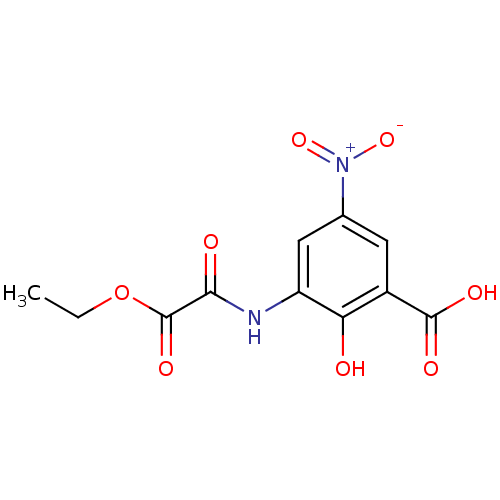 (3-(Ethoxyoxalyl-amino)-2-hydroxy-5-nitro-benzoic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at time 2 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025980 (7-Hydroxy-4-oxo-4H-chromene-2-carboxylic acid benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase (HPAR) | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025972 (7-Hydroxy-4-oxo-4a,8a-dihydro-4H-chromene-2-carbox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in 30 -70% (NH4)2SO4. | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025979 (3-(Ethoxyoxalyl-amino)-2-hydroxy-5-nitro-benzoic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at at time 2 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025976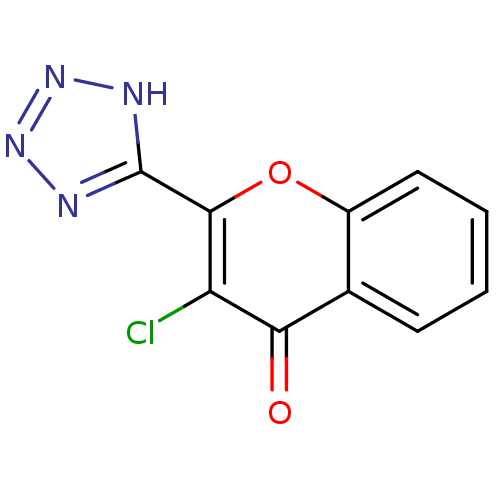 (3-Chloro-2-(1H-tetrazol-5-yl)-chromen-4-one | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at day 7 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025977 (7-Hydroxy-4-oxo-4H-chromene-2-carboxylic acid isop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase (HPAR) | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025975 (3-Hydroxy-11-oxo-11H-pyrido[2,1-b]quinazoline-8-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in 30 -70% (NH4)2SO4. | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025979 (3-(Ethoxyoxalyl-amino)-2-hydroxy-5-nitro-benzoic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at day 1 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025972 (7-Hydroxy-4-oxo-4a,8a-dihydro-4H-chromene-2-carbox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at day 7 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at day 8 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025973 (7-Hydroxy-4-oxo-4H-chromene-2-carboxylic acid ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase (HPAR) | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025975 (3-Hydroxy-11-oxo-11H-pyrido[2,1-b]quinazoline-8-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at day 7 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025972 (7-Hydroxy-4-oxo-4a,8a-dihydro-4H-chromene-2-carbox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in 30 -70% (NH4)2SO4. | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16415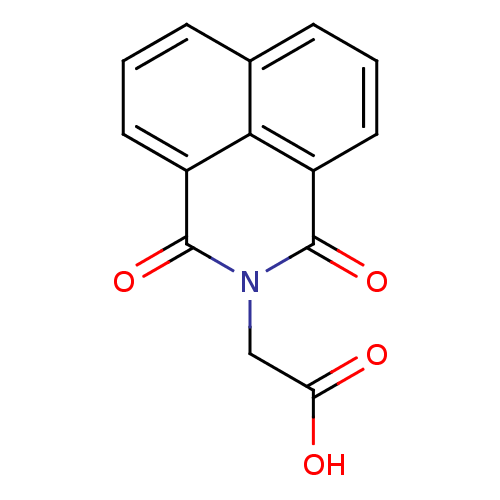 ((1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at time 1 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025974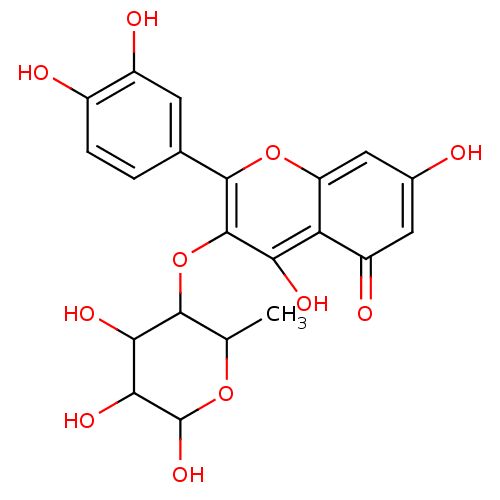 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(4,5,6-tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at day 7 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at day 7 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025975 (3-Hydroxy-11-oxo-11H-pyrido[2,1-b]quinazoline-8-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at at time 2 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025979 (3-(Ethoxyoxalyl-amino)-2-hydroxy-5-nitro-benzoic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at day 8 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025975 (3-Hydroxy-11-oxo-11H-pyrido[2,1-b]quinazoline-8-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at day 1 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025979 (3-(Ethoxyoxalyl-amino)-2-hydroxy-5-nitro-benzoic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at day 2 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025974 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(4,5,6-tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at day 8 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025974 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(4,5,6-tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at day 7 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025974 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(4,5,6-tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at day 1 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025974 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(4,5,6-tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at day 1 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025975 (3-Hydroxy-11-oxo-11H-pyrido[2,1-b]quinazoline-8-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at day 2 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16415 ((1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at time 1 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16415 ((1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in 30 -70% (NH4)2SO4. | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025976 (3-Chloro-2-(1H-tetrazol-5-yl)-chromen-4-one | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Sepharose 4B column at time 1 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025976 (3-Chloro-2-(1H-tetrazol-5-yl)-chromen-4-one | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in 30 -70% (NH4)2SO4. | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16415 ((1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at at day 2 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16415 ((1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in 30 -70% (NH4)2SO4. | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025976 (3-Chloro-2-(1H-tetrazol-5-yl)-chromen-4-one | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in 30 -70% (NH4)2SO4. | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50025976 (3-Chloro-2-(1H-tetrazol-5-yl)-chromen-4-one | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at day 2 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at day 8 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16312 ((4S)-6-fluoro-2,3-dihydrospiro[1-benzopyran-4,4'-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 2.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human placental aldose reductase in Orange A column at day 8 | J Med Chem 28: 841-9 (1985) BindingDB Entry DOI: 10.7270/Q2J965DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||