

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50066870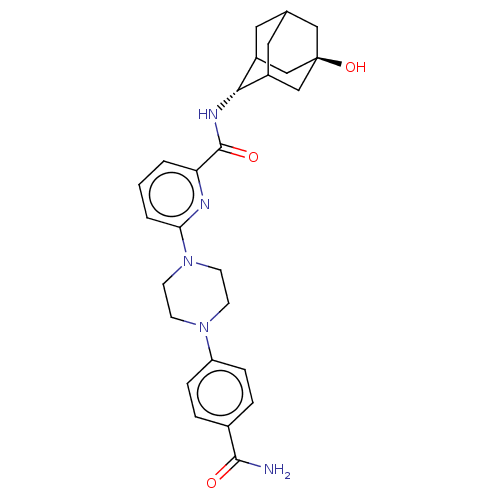 (CHEMBL3401672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells using NADPH assessed as conversion of cortisone to cortisol by cell-based assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489343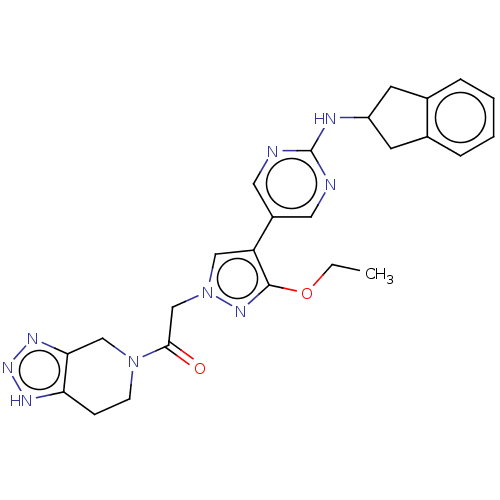 (Example 10-1 | US10961242, Compound 61 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50066894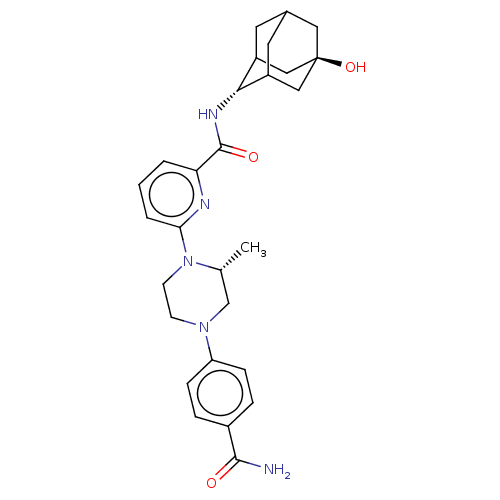 (CHEMBL3401683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells using NADPH assessed as conversion of cortisone to cortisol by cell-based assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489360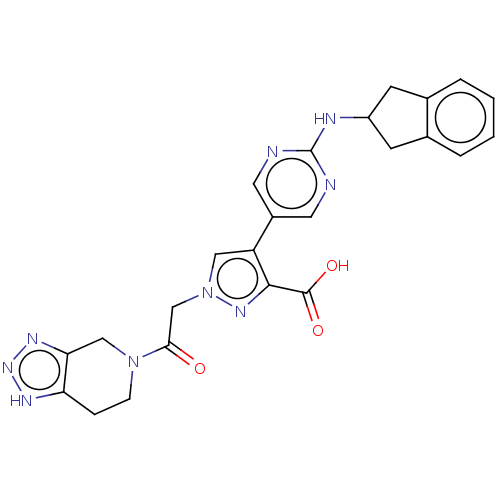 (Example 10-15 | US10961242, Compound 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489343 (Example 10-1 | US10961242, Compound 61 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM582922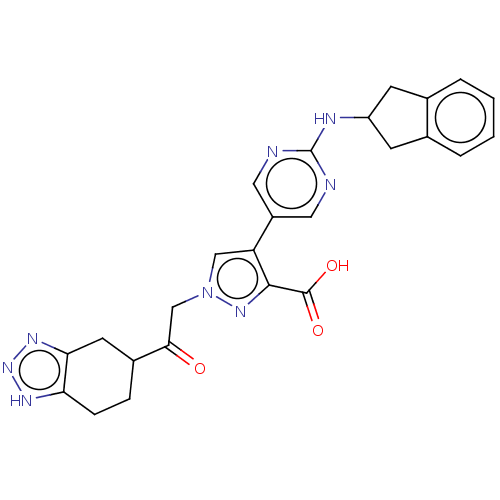 (Acid | US11548883, Compound 75) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489368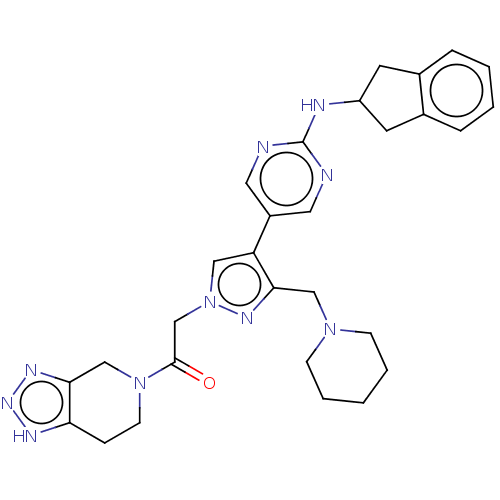 (Example 12-2 | US10961242, Compound 81 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489367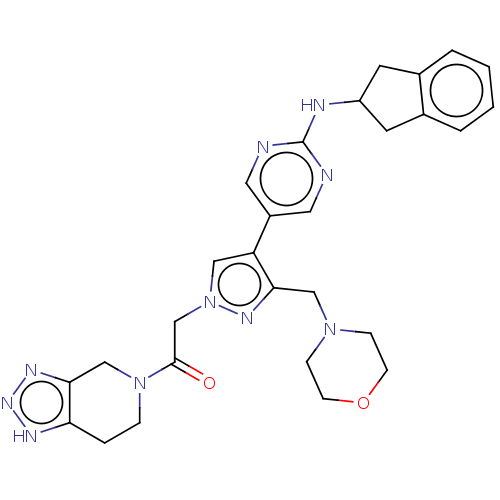 (Example 12-1 | US10961242, Compound 80 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489369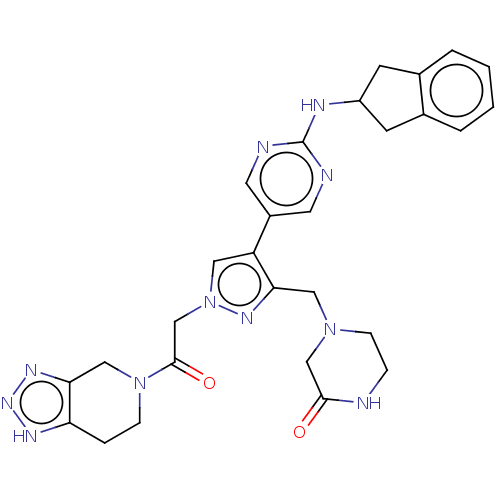 (Example 12-3 | US10961242, Compound 82 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489368 (Example 12-2 | US10961242, Compound 81 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489347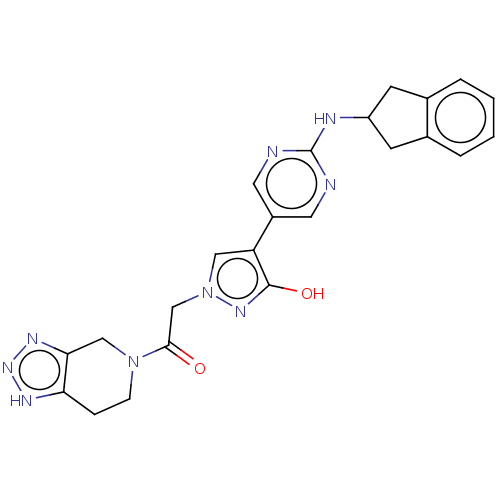 (Example 10-5 | US10961242, Compound 65 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489367 (Example 12-1 | US10961242, Compound 80 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489369 (Example 12-3 | US10961242, Compound 82 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489347 (Example 10-5 | US10961242, Compound 65 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50066871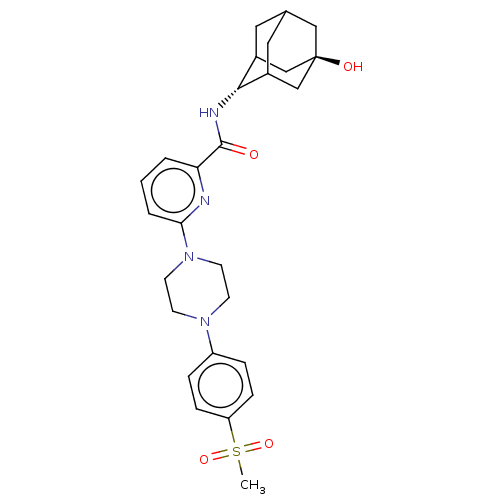 (CHEMBL3401671) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells using NADPH assessed as conversion of cortisone to cortisol by cell-based assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50550263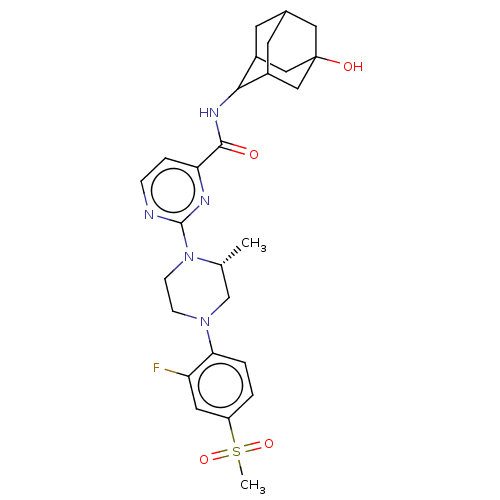 (CHEMBL4748750) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse 11beta-HSD1 expressed in microsomes | Citation and Details BindingDB Entry DOI: 10.7270/Q27P930B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489366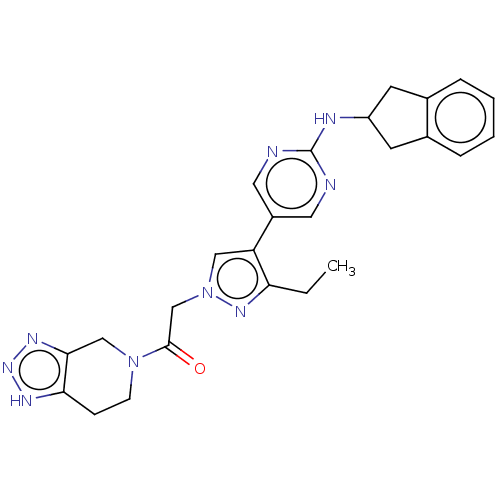 (Example 11-2 | US10961242, Compound 79 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489366 (Example 11-2 | US10961242, Compound 79 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489357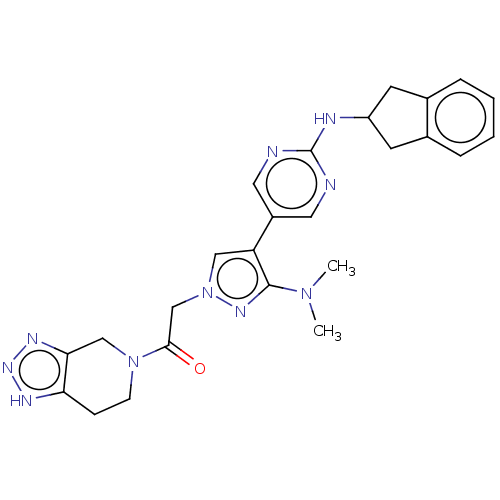 (Example 10-14 | US10961242, Compound 74 | US115488...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489357 (Example 10-14 | US10961242, Compound 74 | US115488...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50060496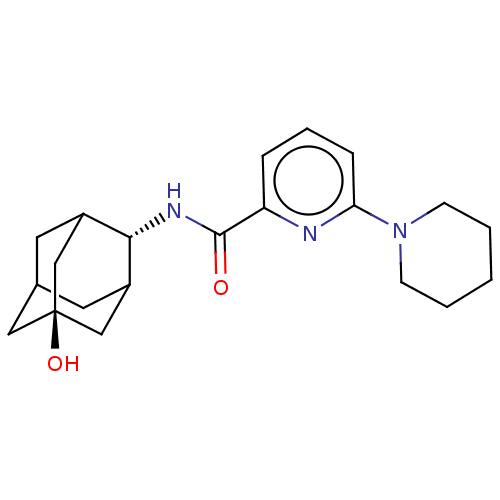 (CHEMBL3394399) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Republic of Korea; Research Institute of Pharmaceutical Science and College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in HEK293 cell microsomal fraction | Bioorg Med Chem Lett 25: 695-700 (2015) Article DOI: 10.1016/j.bmcl.2014.11.074 BindingDB Entry DOI: 10.7270/Q24B32ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489342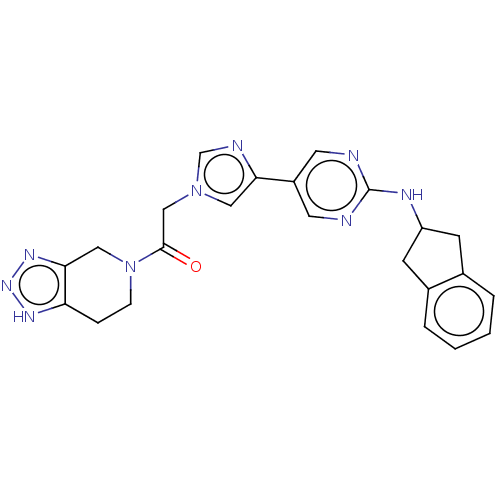 (Example 9 | US10961242, Compound 60) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50060501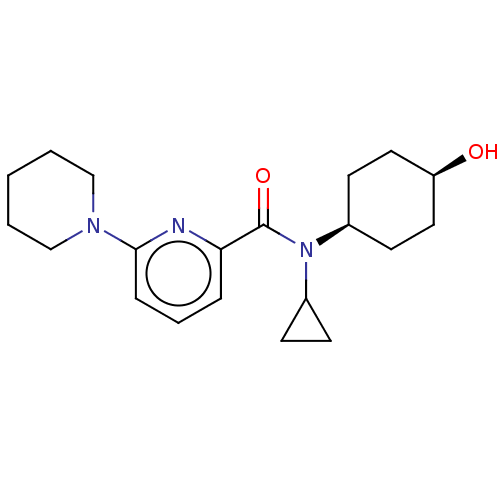 (CHEMBL3394394) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Republic of Korea; Research Institute of Pharmaceutical Science and College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in HEK293 cell microsomal fraction | Bioorg Med Chem Lett 25: 695-700 (2015) Article DOI: 10.1016/j.bmcl.2014.11.074 BindingDB Entry DOI: 10.7270/Q24B32ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM582907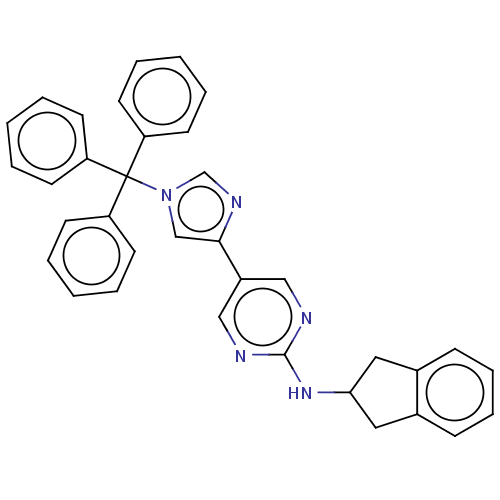 (2-(4-{2-[(2,3-dihydro-1H-inden-2-yl)amino]pyrimidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489351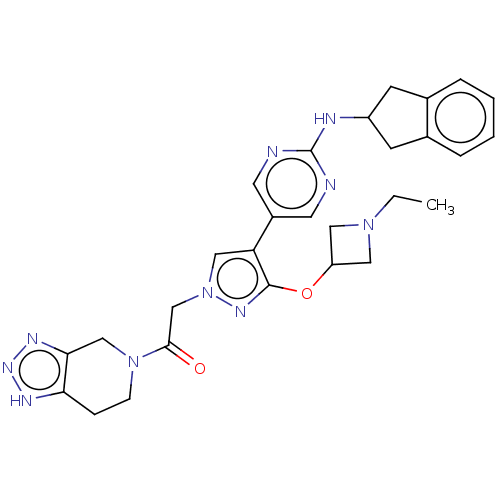 (Example 10-8 | US10961242, Compound 68 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489351 (Example 10-8 | US10961242, Compound 68 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50066887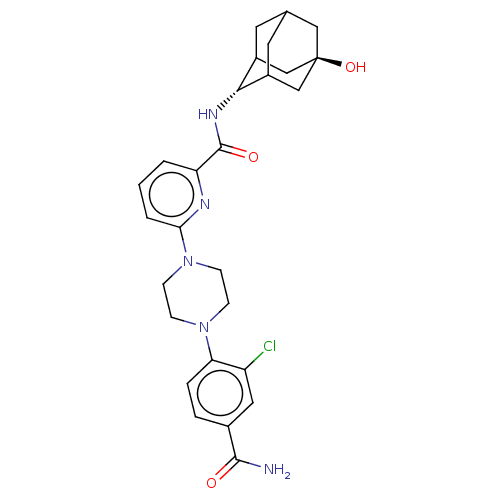 (CHEMBL3401676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells using NADPH assessed as conversion of cortisone to cortisol by cell-based assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50066892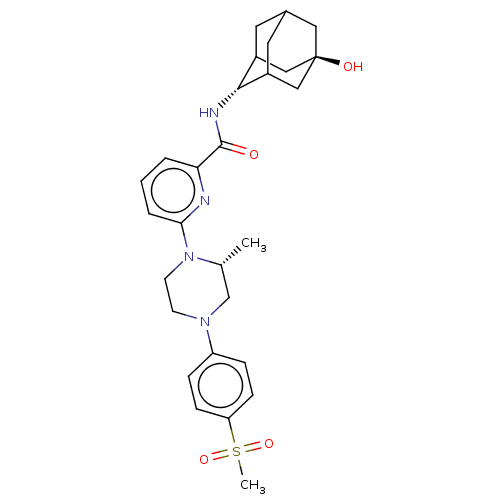 (CHEMBL3401681) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 using microsomal fraction and NADPH assessed as conversion of cortisone to cortisol by biochemical enzyme assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489348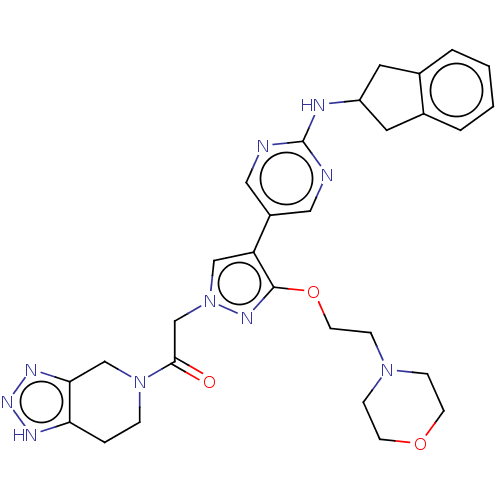 (Example 10-6 | US10961242, Compound 66 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50060491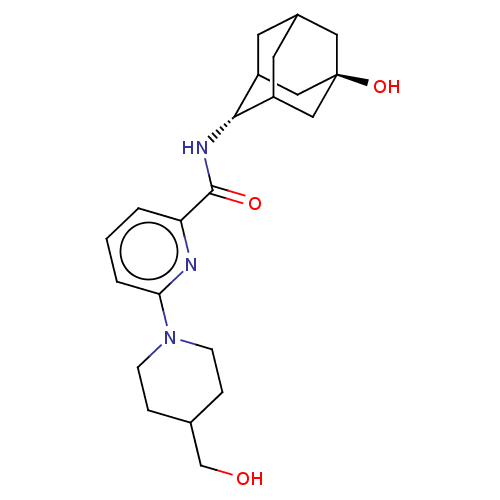 (CHEMBL3394404) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Republic of Korea; Research Institute of Pharmaceutical Science and College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in HEK293 cell microsomal fraction | Bioorg Med Chem Lett 25: 695-700 (2015) Article DOI: 10.1016/j.bmcl.2014.11.074 BindingDB Entry DOI: 10.7270/Q24B32ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489348 (Example 10-6 | US10961242, Compound 66 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50066874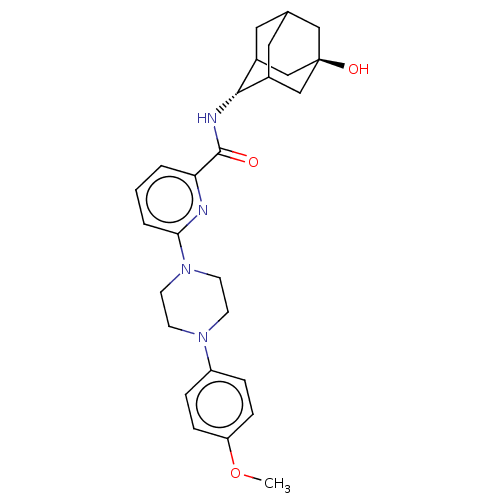 (CHEMBL3401668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 using microsomal fraction and NADPH assessed as conversion of cortisone to cortisol by biochemical enzyme assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489315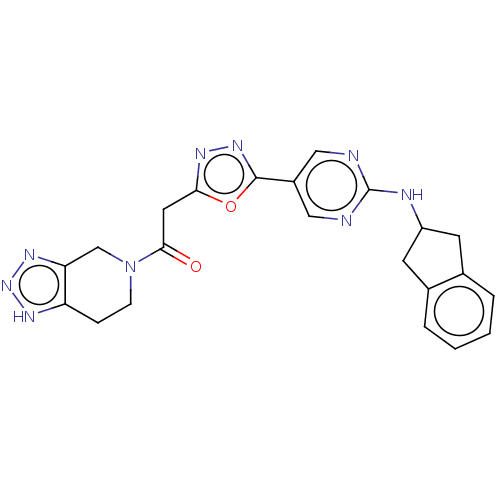 (Example 6-1 | US10961242, Compound 33 | US11548883...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489316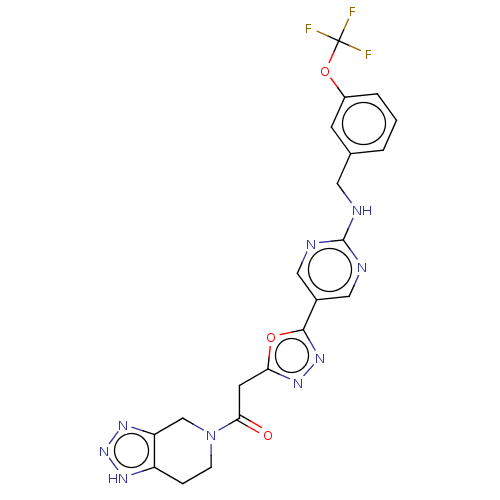 (Example 6-2 | US10961242, Compound 34 | US11548883...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489332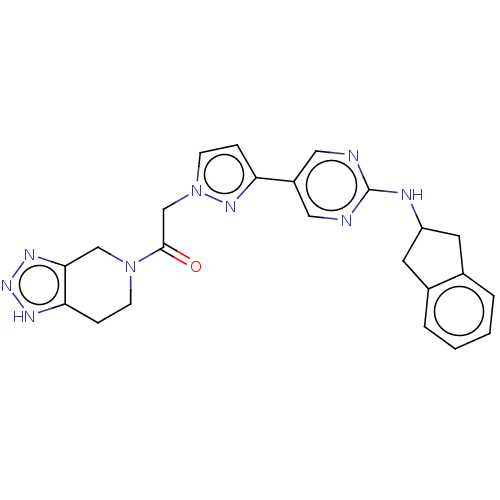 ( 2-(5-{2-[(2,3-dihydro-1H-inden-2-yl)amino]pyrimid...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489337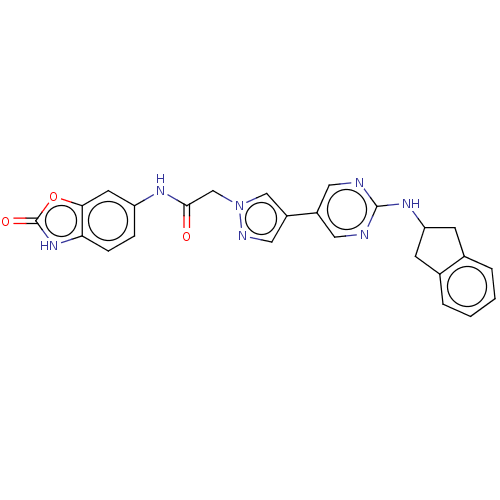 (Example 8-2 | US10961242, Compound 57 | US11548883...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489316 (Example 6-2 | US10961242, Compound 34 | US11548883...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489337 (Example 8-2 | US10961242, Compound 57 | US11548883...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489292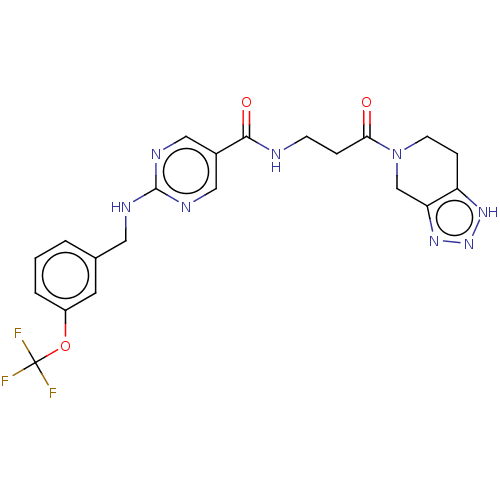 (Example 1-2 | US10961242, Compound 2 | US11548883,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489292 (Example 1-2 | US10961242, Compound 2 | US11548883,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489386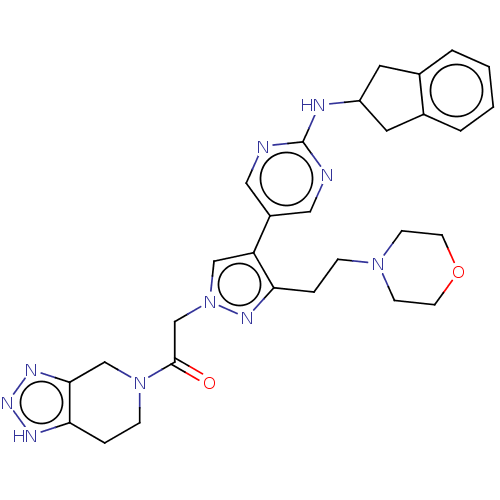 (Example 12-14 | US10961242, Compound 93 | US115488...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489370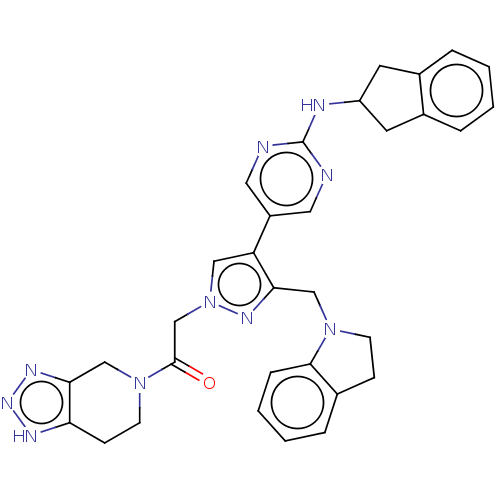 (Example 12-4 | US10961242, Compound 83 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489370 (Example 12-4 | US10961242, Compound 83 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489386 (Example 12-14 | US10961242, Compound 93 | US115488...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50066873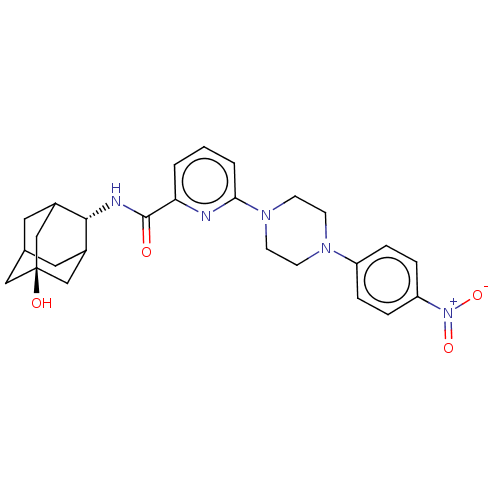 (CHEMBL3401669) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD2 using microsomal fraction and NADPH assessed as conversion of cortisone to cortisol by biochemical enzyme assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489372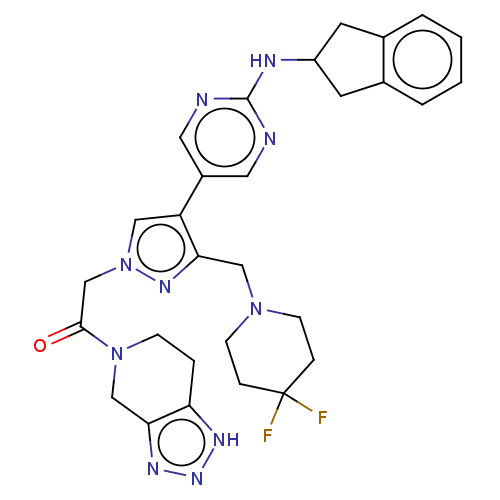 (Example 12-6 | US10961242, Compound 85 | US1154888...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489336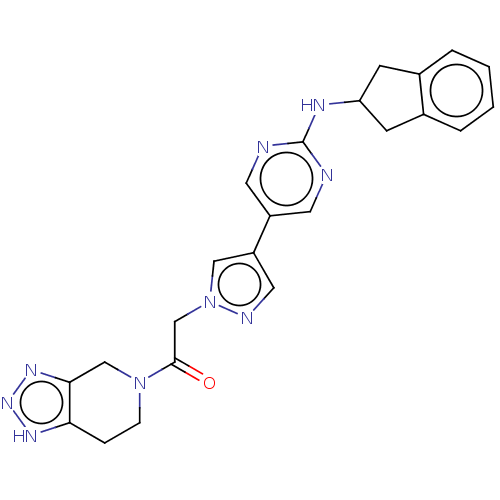 (Example 8-1 | US10961242, Compound 56 | US11548883...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
LegoChem Biosciences, Inc. US Patent | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | US Patent US10961242 (2021) BindingDB Entry DOI: 10.7270/Q27084JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489301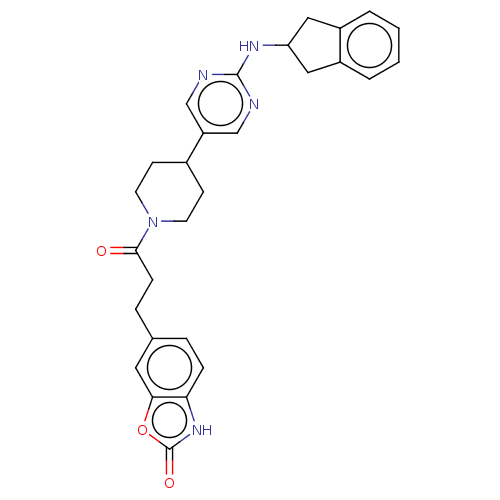 (Example 2-5 | US10961242, Compound 16 | US11548883...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM489336 (Example 8-1 | US10961242, Compound 56 | US11548883...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Two-fold dilution of each test compound solution (10 μM, 100% dimethyl sulfoxide) is carried out on 96-well V bottom plate (Costar 3363). After ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2F76HDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50066880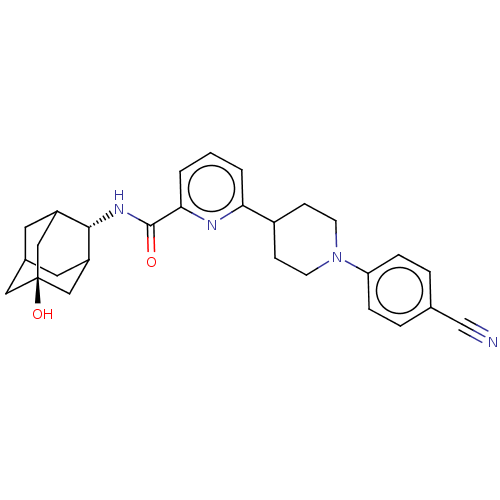 (CHEMBL3401662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 using microsomal fraction and NADPH assessed as conversion of cortisone to cortisol by biochemical enzyme assay | Bioorg Med Chem Lett 25: 1679-83 (2015) Article DOI: 10.1016/j.bmcl.2015.03.003 BindingDB Entry DOI: 10.7270/Q2X3504Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 393 total ) | Next | Last >> |