 Found 6 hits with Last Name = 'reed' and Initial = 'jk'
Found 6 hits with Last Name = 'reed' and Initial = 'jk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50289150

(Acetic acid (7E,11E)-(1S,2R,4R,14S,15R)-4,8,12-tri...)Show SMILES CC(=O)O[C@H]1C\C(C)=C\CC\C(C)=C\CC[C@@]2(C)O[C@@H]2[C@H]2OC(=O)C(=C)[C@H]12 |t:7,12| Show InChI InChI=1S/C22H30O5/c1-13-8-6-9-14(2)12-17(25-16(4)23)18-15(3)21(24)26-19(18)20-22(5,27-20)11-7-10-13/h9-10,17-20H,3,6-8,11-12H2,1-2,4-5H3/b13-10+,14-9+/t17-,18+,19-,20+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Kinetic parameter for inihibiton of farnesyl protein transferase |
Bioorg Med Chem Lett 6: 909-912 (1996)
Article DOI: 10.1016/0960-894X(96)00142-4
BindingDB Entry DOI: 10.7270/Q2VT1S3W |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50289150

(Acetic acid (7E,11E)-(1S,2R,4R,14S,15R)-4,8,12-tri...)Show SMILES CC(=O)O[C@H]1C\C(C)=C\CC\C(C)=C\CC[C@@]2(C)O[C@@H]2[C@H]2OC(=O)C(=C)[C@H]12 |t:7,12| Show InChI InChI=1S/C22H30O5/c1-13-8-6-9-14(2)12-17(25-16(4)23)18-15(3)21(24)26-19(18)20-22(5,27-20)11-7-10-13/h9-10,17-20H,3,6-8,11-12H2,1-2,4-5H3/b13-10+,14-9+/t17-,18+,19-,20+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inihibtion of recombinant human farnesyl protein transferase |
Bioorg Med Chem Lett 6: 909-912 (1996)
Article DOI: 10.1016/0960-894X(96)00142-4
BindingDB Entry DOI: 10.7270/Q2VT1S3W |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50478457
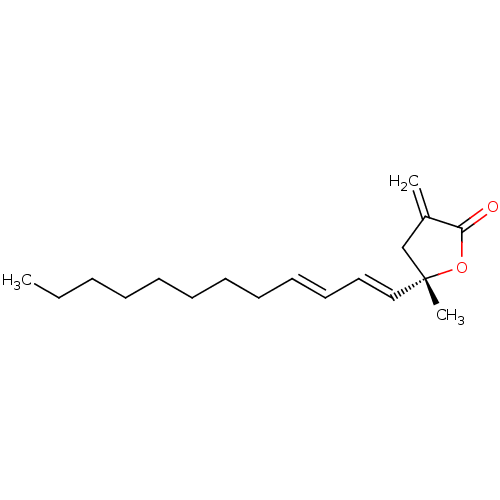
(PLAKOLIDE A)Show InChI InChI=1S/C18H28O2/c1-4-5-6-7-8-9-10-11-12-13-14-18(3)15-16(2)17(19)20-18/h11-14H,2,4-10,15H2,1,3H3/b12-11+,14-13+/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
Harbor Branch Oceanographic Institution
Curated by ChEMBL
| Assay Description
Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells by Griess method |
J Nat Prod 67: 110-1 (2004)
Article DOI: 10.1021/np030294c
BindingDB Entry DOI: 10.7270/Q2VQ35FN |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50316411
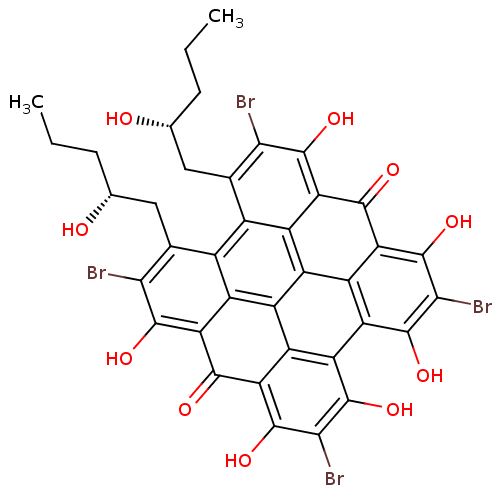
(CHEMBL1094578 | gymnochrome F)Show SMILES CCC[C@@H](O)Cc1c(Br)c(O)c2c3c1c1c(C[C@H](O)CCC)c(Br)c(O)c4c1c1c3c3c(c(O)c(Br)c(O)c3c2=O)c2c(O)c(Br)c(O)c(c12)c4=O |r| Show InChI InChI=1S/C38H28Br4O10/c1-3-5-9(43)7-11-13-14-12(8-10(44)6-4-2)28(40)36(50)24-16(14)18-17-15(13)23(35(49)27(11)39)31(45)25-19(17)21(33(47)29(41)37(25)51)22-20(18)26(32(24)46)38(52)30(42)34(22)48/h9-10,43-44,47-52H,3-8H2,1-2H3/t9-,10-/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harbor Branch Oceanographic Institute at Florida Atlantic University
Curated by ChEMBL
| Assay Description
Inhibition of MCL1 binding to Bak by FRET assay |
J Nat Prod 73: 712-5 (2010)
Article DOI: 10.1021/np900526y
BindingDB Entry DOI: 10.7270/Q2VQ32TB |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50289150

(Acetic acid (7E,11E)-(1S,2R,4R,14S,15R)-4,8,12-tri...)Show SMILES CC(=O)O[C@H]1C\C(C)=C\CC\C(C)=C\CC[C@@]2(C)O[C@@H]2[C@H]2OC(=O)C(=C)[C@H]12 |t:7,12| Show InChI InChI=1S/C22H30O5/c1-13-8-6-9-14(2)12-17(25-16(4)23)18-15(3)21(24)26-19(18)20-22(5,27-20)11-7-10-13/h9-10,17-20H,3,6-8,11-12H2,1-2,4-5H3/b13-10+,14-9+/t17-,18+,19-,20+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inihibtory activity against recombinant human Geranylgeranyl transferase type I |
Bioorg Med Chem Lett 6: 909-912 (1996)
Article DOI: 10.1016/0960-894X(96)00142-4
BindingDB Entry DOI: 10.7270/Q2VT1S3W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50316410

(CHEMBL1094577 | gymnochrome E)Show SMILES CCC[C@@H](O)Cc1c(Br)c(O)c2c3c1c1c(C[C@H](C)O)c(Br)c(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O |r| Show InChI InChI=1S/C36H26Br2O10/c1-3-4-10(40)6-12-18-17-11(5-9(2)39)31(37)35(47)29-23(17)27-25-19(13(41)7-15(43)21(25)33(29)45)20-14(42)8-16(44)22-26(20)28(27)24(18)30(34(22)46)36(48)32(12)38/h7-10,39-44,47-48H,3-6H2,1-2H3/t9-,10+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harbor Branch Oceanographic Institute at Florida Atlantic University
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 assessed as [3H]acetate release after 90 mins by scintillation counting |
J Nat Prod 73: 712-5 (2010)
Article DOI: 10.1021/np900526y
BindingDB Entry DOI: 10.7270/Q2VQ32TB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data