 Found 244 hits with Last Name = 'yu' and Initial = 'jl'
Found 244 hits with Last Name = 'yu' and Initial = 'jl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM370555
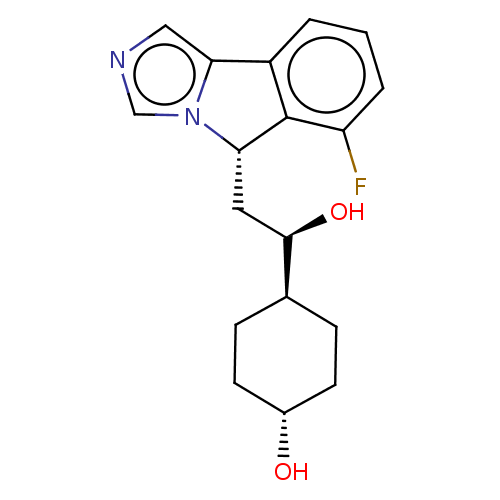
((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...)Show SMILES O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 |r,wU:19.22,wD:3.2,1.0,16.19,(-.99,-3.03,;-.22,-1.7,;-.99,-.37,;-2.53,-.37,;-3.37,.92,;-4.85,.53,;-5.94,1.61,;-5.54,3.1,;-4.06,3.5,;-2.97,2.41,;-1.48,2.81,;-4.93,-1.01,;-5.9,-2.21,;-5.06,-3.5,;-3.58,-3.1,;-3.5,-1.56,;1.32,-1.7,;2.09,-3.03,;3.63,-3.03,;4.4,-1.7,;5.94,-1.7,;3.63,-.37,;2.09,-.37,)| Show InChI InChI=1S/C18H21FN2O2/c19-14-3-1-2-13-16-9-20-10-21(16)15(18(13)14)8-17(23)11-4-6-12(22)7-5-11/h1-3,9-12,15,17,22-23H,4-8H2/t11-,12-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50561901
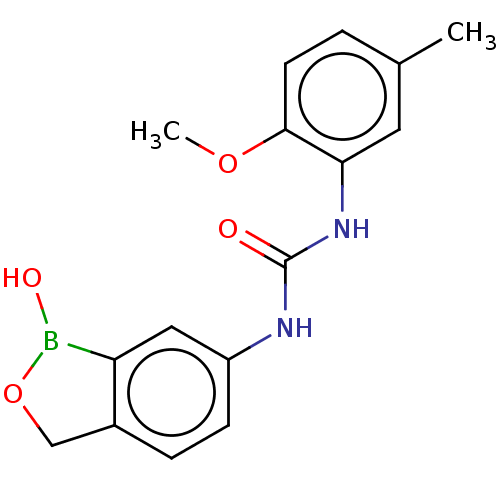
(CHEMBL4746021) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50279661

(CHEMBL4166065)Show InChI InChI=1S/C15H12BF3N2O2S/c17-15(18,19)10-2-5-11(6-3-10)20-14(24)21-12-4-1-9-8-23-16(22)13(9)7-12/h1-7,22H,8H2,(H2,20,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50294581
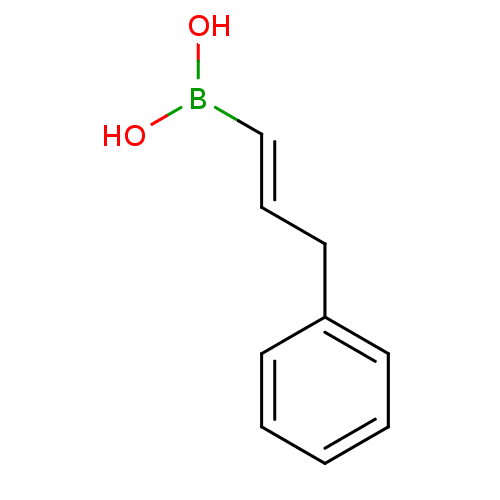
(3-phenylprop-1-enylboronic acid | CHEMBL539140)Show InChI InChI=1S/C9H11BO2/c11-10(12)8-4-7-9-5-2-1-3-6-9/h1-6,8,11-12H,7H2/b8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50561901

(CHEMBL4746021) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50279661

(CHEMBL4166065)Show InChI InChI=1S/C15H12BF3N2O2S/c17-15(18,19)10-2-5-11(6-3-10)20-14(24)21-12-4-1-9-8-23-16(22)13(9)7-12/h1-7,22H,8H2,(H2,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50561901

(CHEMBL4746021) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606592

(CHEMBL5219838) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50200540

(CHEMBL3972619)Show InChI InChI=1S/C14H12BrN3O/c15-10-5-13(12-8-17-18-14(12)6-10)16-7-9-1-3-11(19)4-2-9/h1-6,8,16,19H,7H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50561901

(CHEMBL4746021) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 414 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50279661

(CHEMBL4166065)Show InChI InChI=1S/C15H12BF3N2O2S/c17-15(18,19)10-2-5-11(6-3-10)20-14(24)21-12-4-1-9-8-23-16(22)13(9)7-12/h1-7,22H,8H2,(H2,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50279661

(CHEMBL4166065)Show InChI InChI=1S/C15H12BF3N2O2S/c17-15(18,19)10-2-5-11(6-3-10)20-14(24)21-12-4-1-9-8-23-16(22)13(9)7-12/h1-7,22H,8H2,(H2,20,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50200540

(CHEMBL3972619)Show InChI InChI=1S/C14H12BrN3O/c15-10-5-13(12-8-17-18-14(12)6-10)16-7-9-1-3-11(19)4-2-9/h1-6,8,16,19H,7H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50606592

(CHEMBL5219838) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50207089
(D-1-Methyltryptophan | D-1MT | Indoximod)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Arginase-1
(Rattus norvegicus) | BDBM50354832

(CHEMBL1834160)Show InChI InChI=1S/C7H13NO3/c8-6(7(10)11)4-2-1-3-5-9/h5-6H,1-4,8H2,(H,10,11)/t6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-1
(Bos taurus) | BDBM50608139

(CHEMBL5287570) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50316603
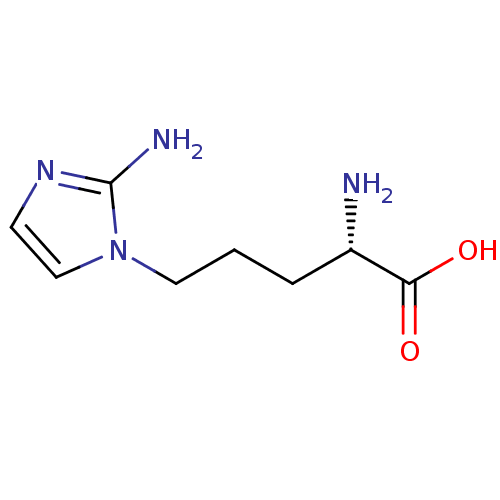
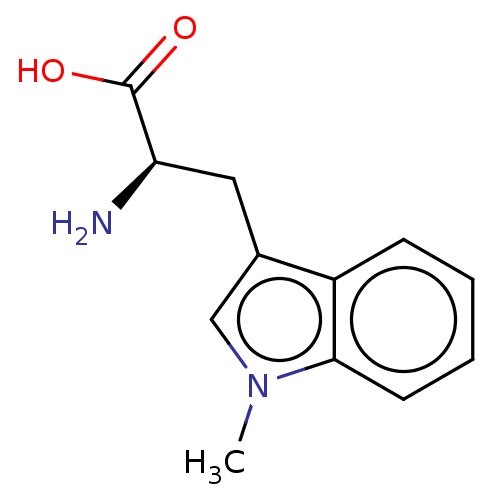
(2-(S)-amino-5-(2-aminoimidazol-1-yl)pentanoic acid...)Show InChI InChI=1S/C8H14N4O2/c9-6(7(13)14)2-1-4-12-5-3-11-8(12)10/h3,5-6H,1-2,4,9H2,(H2,10,11)(H,13,14)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| | 3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM589753

(US11559538, Example 16 | US11559538, Example 208 |...) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50608137
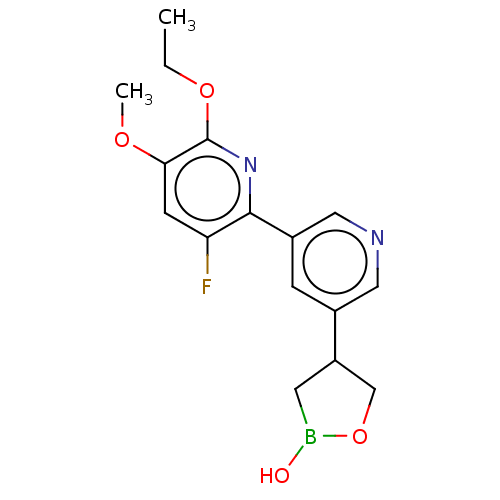
(CHEMBL5268265) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM589895

(US11559538, Example 147 | US11559538, Example 148) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50568657
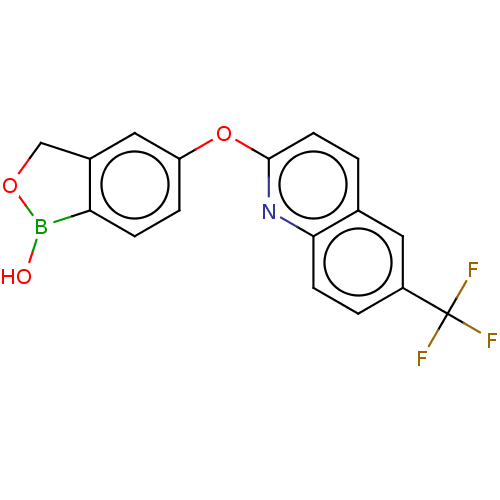
(CHEMBL4872870) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50608132

(CHEMBL5290174) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50285416
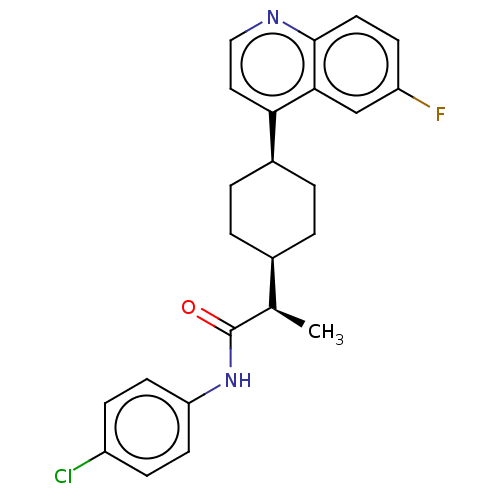
(CHEMBL4161733)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:18.21,1.0,wD:4.7,(66.16,-9.22,;66.17,-7.68,;66.16,-6.15,;67.5,-5.38,;68.84,-6.15,;68.83,-7.69,;67.5,-8.46,;70.18,-5.39,;71.5,-6.16,;72.84,-5.4,;72.84,-3.85,;71.5,-3.08,;71.5,-1.55,;70.17,-.79,;68.84,-1.56,;67.5,-.8,;68.85,-3.09,;70.18,-3.85,;64.83,-8.46,;64.84,-10,;63.5,-7.69,;63.49,-6.14,;62.16,-8.46,;60.83,-7.69,;59.49,-8.47,;58.15,-7.69,;58.16,-6.14,;56.82,-5.38,;59.49,-5.38,;60.83,-6.14,)| Show InChI InChI=1S/C24H24ClFN2O/c1-15(24(29)28-20-9-6-18(25)7-10-20)16-2-4-17(5-3-16)21-12-13-27-23-11-8-19(26)14-22(21)23/h6-17H,2-5H2,1H3,(H,28,29)/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-lactamase TEM
(Escherichia coli) | BDBM50339145

(CHEMBL1689063 | trans-7-oxo-6-(sulfooxy)-1,6-diaza...)Show SMILES NC(=O)[C@@H]1CC[C@@H]2CN1C(=O)N2OS(O)(=O)=O |r| Show InChI InChI=1S/C7H11N3O6S/c8-6(11)5-2-1-4-3-9(5)7(12)10(4)16-17(13,14)15/h4-5H,1-3H2,(H2,8,11)(H,13,14,15)/t4-,5+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of bacterial Beta-lactamase TEM-1 (24 - 286) (unknown origin) expressed in Escherichia coli Transetta (DE3) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127956
BindingDB Entry DOI: 10.7270/Q2B85CX1 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a/A2b
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of binding to Adenosine-2 receptor using [3H]NECA in rat striatum |
J Med Chem 31: 1014-20 (1988)
BindingDB Entry DOI: 10.7270/Q2T43S4W |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50322726

(2-[(4-{[3-(4-Fluorobenzyl)-2,4-dioxo-1,3-thiazolan...)Show SMILES OB(O)c1ccccc1COc1ccc(\C=C2/SC(=O)N(Cc3ccc(F)cc3)C2=O)cc1 Show InChI InChI=1S/C24H19BFNO5S/c26-19-9-5-17(6-10-19)14-27-23(28)22(33-24(27)29)13-16-7-11-20(12-8-16)32-15-18-3-1-2-4-21(18)25(30)31/h1-13,30-31H,14-15H2/b22-13- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50347722
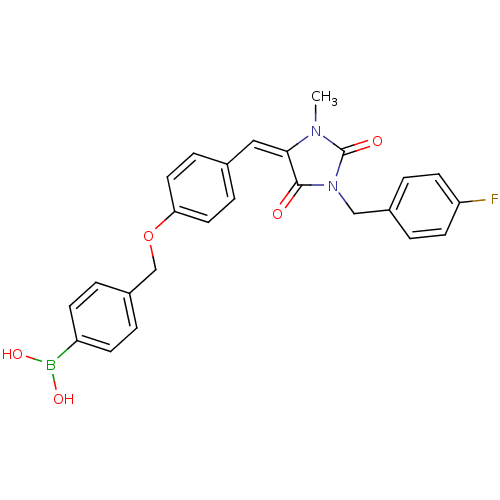
(CHEMBL1802517)Show SMILES CN1C(=O)N(Cc2ccc(F)cc2)C(=O)\C1=C/c1ccc(OCc2ccc(cc2)B(O)O)cc1 Show InChI InChI=1S/C25H22BFN2O5/c1-28-23(24(30)29(25(28)31)15-18-4-10-21(27)11-5-18)14-17-6-12-22(13-7-17)34-16-19-2-8-20(9-3-19)26(32)33/h2-14,32-33H,15-16H2,1H3/b23-14+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a/A2b
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50023492

(5-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazo...)Show InChI InChI=1S/C13H9N5O2/c14-13-15-9-4-3-7(19)6-8(9)12-16-11(17-18(12)13)10-2-1-5-20-10/h1-6,19H,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of binding to Adenosine-2 receptor using [3H]NECA in rat striatum |
J Med Chem 31: 1014-20 (1988)
BindingDB Entry DOI: 10.7270/Q2T43S4W |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50322727
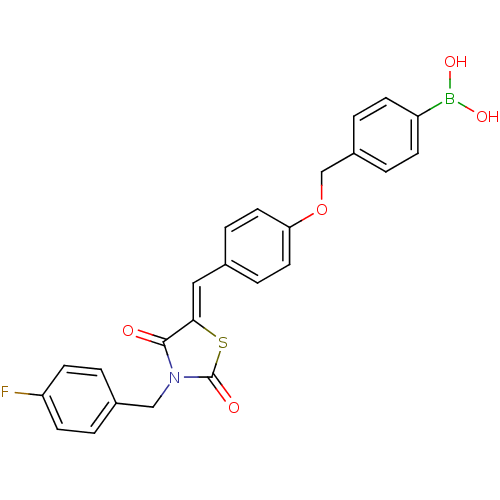
(4-[(4-{[3-(4-Fluorobenzyl)-2,4-dioxo-1,3-thiazolan...)Show SMILES OB(O)c1ccc(COc2ccc(\C=C3/SC(=O)N(Cc4ccc(F)cc4)C3=O)cc2)cc1 Show InChI InChI=1S/C24H19BFNO5S/c26-20-9-3-17(4-10-20)14-27-23(28)22(33-24(27)29)13-16-5-11-21(12-6-16)32-15-18-1-7-19(8-2-18)25(30)31/h1-13,30-31H,14-15H2/b22-13- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50347723

(CHEMBL1802518)Show SMILES CN1C(=O)N(Cc2ccc(F)cc2)C(=O)\C1=C\c1ccc(OCc2ccc(cc2)B(O)O)cc1 Show InChI InChI=1S/C25H22BFN2O5/c1-28-23(24(30)29(25(28)31)15-18-4-10-21(27)11-5-18)14-17-6-12-22(13-7-17)34-16-19-2-8-20(9-3-19)26(32)33/h2-14,32-33H,15-16H2,1H3/b23-14- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50562497
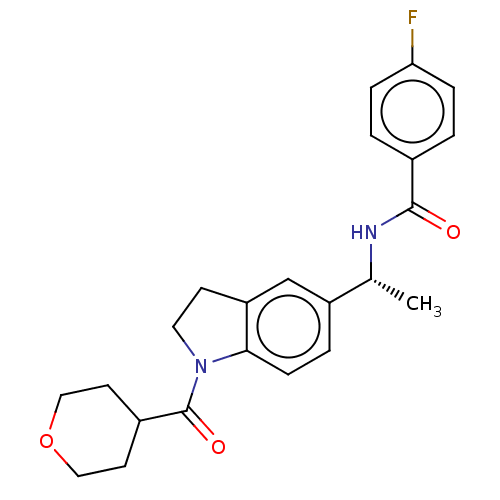
(CHEMBL4778760)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCc2c1)C(=O)C1CCOCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
VIM-1 metallo-beta-lactamase
(Klebsiella pneumoniae) | BDBM50608143

(CHEMBL4463697)Show SMILES NCCN[C@H]1CC[C@H](CC(=O)N[C@H]2Cc3cccc(C(O)=O)c3OB2O)CC1 |r,wU:4.3,12.11,wD:7.7,(5.28,-8.39,;6.61,-9.16,;7.94,-8.39,;9.27,-9.16,;10.61,-8.39,;10.61,-6.85,;11.94,-6.08,;13.27,-6.85,;14.61,-6.08,;15.94,-6.85,;15.94,-8.39,;17.27,-6.08,;18.6,-6.85,;19.94,-6.08,;21.27,-6.85,;22.6,-6.08,;23.94,-6.86,;23.94,-8.39,;22.61,-9.16,;22.61,-10.7,;21.28,-11.48,;23.95,-11.48,;21.27,-8.39,;19.94,-9.16,;18.6,-8.39,;17.27,-9.16,;13.27,-8.39,;11.94,-9.16,)| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50023492

(5-Amino-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazo...)Show InChI InChI=1S/C13H9N5O2/c14-13-15-9-4-3-7(19)6-8(9)12-16-11(17-18(12)13)10-2-1-5-20-10/h1-6,19H,(H2,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of binding towards adenosine A1 receptor using [3H]-N-cyclohexyladenosine ([3H]-CHA) in rat whole brain membranes. |
J Med Chem 31: 1014-20 (1988)
BindingDB Entry DOI: 10.7270/Q2T43S4W |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50590774

(CHEMBL5203175) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50608136

(CHEMBL1093374) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50322725
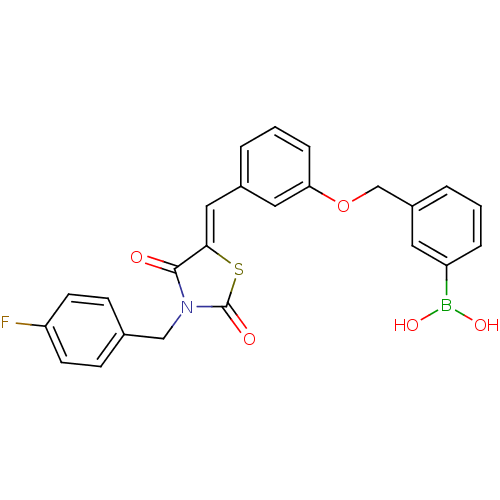
(3-[(3-{[3-(4-Fluorobenzyl)-2,4-dioxo-1,3-thiazolan...)Show SMILES OB(O)c1cccc(COc2cccc(\C=C3/SC(=O)N(Cc4ccc(F)cc4)C3=O)c2)c1 Show InChI InChI=1S/C24H19BFNO5S/c26-20-9-7-16(8-10-20)14-27-23(28)22(33-24(27)29)13-17-3-2-6-21(12-17)32-15-18-4-1-5-19(11-18)25(30)31/h1-13,30-31H,14-15H2/b22-13- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM103575
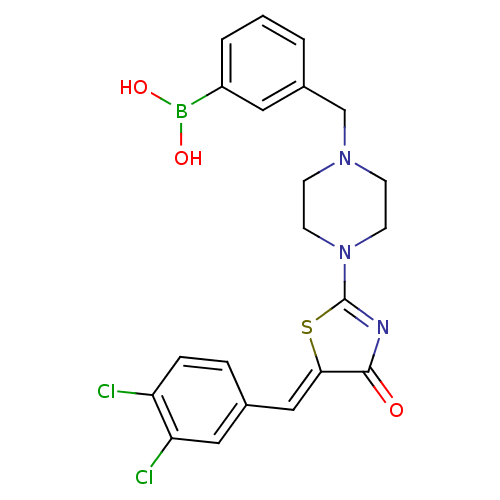
(ATX inhibitor 10 analogue 5 | US8551988, 65)Show SMILES OB(O)c1cccc(CN2CCN(CC2)C2=NC(=O)\C(S2)=C\c2ccc(Cl)c(Cl)c2)c1 |t:16| Show InChI InChI=1S/C21H20BCl2N3O3S/c23-17-5-4-14(11-18(17)24)12-19-20(28)25-21(31-19)27-8-6-26(7-9-27)13-15-2-1-3-16(10-15)22(29)30/h1-5,10-12,29-30H,6-9,13H2/b19-12- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a/A2b
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50023505

(2-Furan-2-yl-9-methoxy-[1,2,4]triazolo[1,5-c]quina...)Show InChI InChI=1S/C14H11N5O2/c1-20-8-4-5-10-9(7-8)13-17-12(11-3-2-6-21-11)18-19(13)14(15)16-10/h2-7H,1H3,(H2,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of binding to Adenosine-2 receptor using [3H]NECA in rat striatum |
J Med Chem 31: 1014-20 (1988)
BindingDB Entry DOI: 10.7270/Q2T43S4W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of binding towards adenosine A1 receptor using [3H]-N-cyclohexyladenosine ([3H]-CHA) in rat whole brain membranes. |
J Med Chem 31: 1014-20 (1988)
BindingDB Entry DOI: 10.7270/Q2T43S4W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50023495
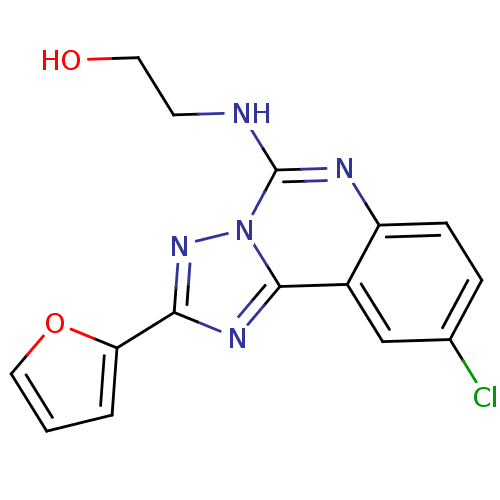
(2-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]qui...)Show InChI InChI=1S/C15H12ClN5O2/c16-9-3-4-11-10(8-9)14-19-13(12-2-1-7-23-12)20-21(14)15(18-11)17-5-6-22/h1-4,7-8,22H,5-6H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of binding towards adenosine A1 receptor using [3H]-N-cyclohexyladenosine ([3H]-CHA) in rat whole brain membranes. |
J Med Chem 31: 1014-20 (1988)
BindingDB Entry DOI: 10.7270/Q2T43S4W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50023500
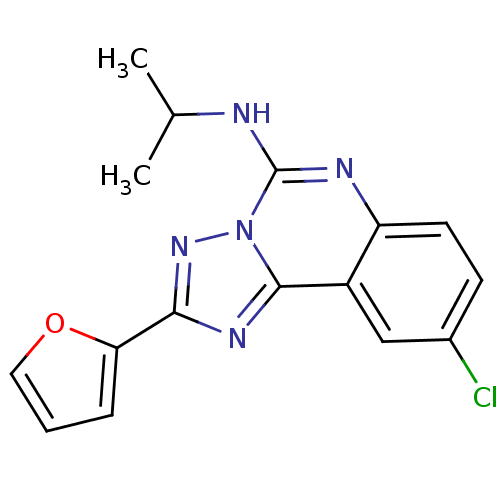
((9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quina...)Show InChI InChI=1S/C16H14ClN5O/c1-9(2)18-16-19-12-6-5-10(17)8-11(12)15-20-14(21-22(15)16)13-4-3-7-23-13/h3-9H,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of binding towards adenosine A1 receptor using [3H]-N-cyclohexyladenosine ([3H]-CHA) in rat whole brain membranes. |
J Med Chem 31: 1014-20 (1988)
BindingDB Entry DOI: 10.7270/Q2T43S4W |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50608131
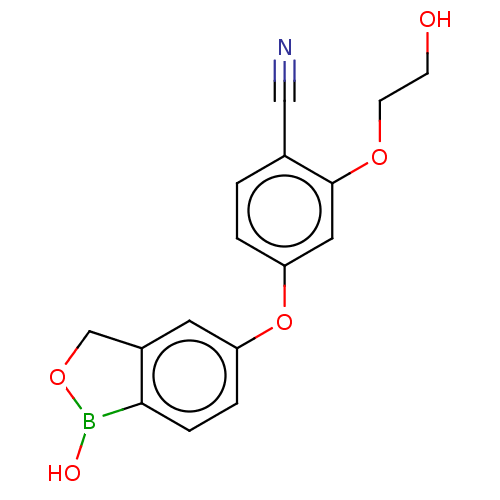
(CHEMBL5278590) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM103577
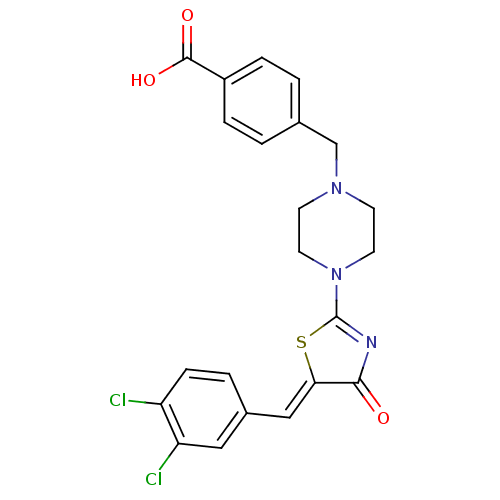
(ATX inhibitor 10 analogue 7)Show SMILES OC(=O)c1ccc(CN2CCN(CC2)C2=NC(=O)\C(S2)=C\c2ccc(Cl)c(Cl)c2)cc1 |t:15| Show InChI InChI=1S/C22H19Cl2N3O3S/c23-17-6-3-15(11-18(17)24)12-19-20(28)25-22(31-19)27-9-7-26(8-10-27)13-14-1-4-16(5-2-14)21(29)30/h1-6,11-12H,7-10,13H2,(H,29,30)/b19-12- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a/A2b
(Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50023496

(2-Furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-yl...)Show InChI InChI=1S/C13H9N5O/c14-13-15-9-5-2-1-4-8(9)12-16-11(17-18(12)13)10-6-3-7-19-10/h1-7H,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of binding to Adenosine-2 receptor using [3H]NECA in rat striatum |
J Med Chem 31: 1014-20 (1988)
BindingDB Entry DOI: 10.7270/Q2T43S4W |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50322724
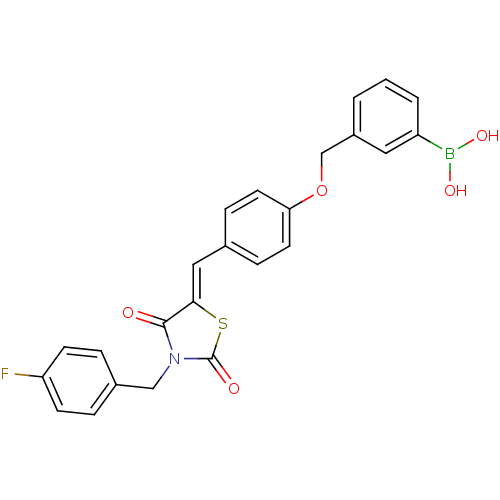
(3-[(4-{[3-(4-Fluorobenzyl)-2,4-dioxo-1,3-thiazolan...)Show SMILES OB(O)c1cccc(COc2ccc(\C=C3/SC(=O)N(Cc4ccc(F)cc4)C3=O)cc2)c1 Show InChI InChI=1S/C24H19BFNO5S/c26-20-8-4-17(5-9-20)14-27-23(28)22(33-24(27)29)13-16-6-10-21(11-7-16)32-15-18-2-1-3-19(12-18)25(30)31/h1-13,30-31H,14-15H2/b22-13- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50590774

(CHEMBL5203175) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50511658
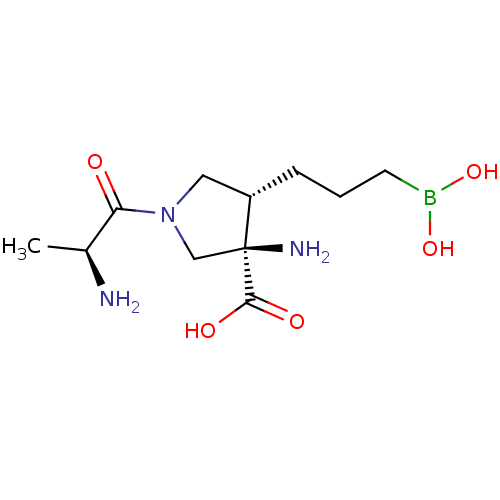
(Cb-1158 | INCB-001158 | Incb 001158 | Incb001158 |...)Show SMILES C[C@H](N)C(=O)N1C[C@H](CCCB(O)O)[C@@](N)(C1)C(O)=O |r| Show InChI InChI=1S/C11H22BN3O5/c1-7(13)9(16)15-5-8(3-2-4-12(19)20)11(14,6-15)10(17)18/h7-8,19-20H,2-6,13-14H2,1H3,(H,17,18)/t7-,8-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50322728

(4-[(3-{[3-(4-Fluorobenzyl)-2,4-dioxo-1,3-thiazolan...)Show SMILES OB(O)c1ccc(COc2cccc(\C=C3/SC(=O)N(Cc4ccc(F)cc4)C3=O)c2)cc1 Show InChI InChI=1S/C24H19BFNO5S/c26-20-10-6-16(7-11-20)14-27-23(28)22(33-24(27)29)13-18-2-1-3-21(12-18)32-15-17-4-8-19(9-5-17)25(30)31/h1-13,30-31H,14-15H2/b22-13- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data