 Found 316 hits with Last Name = 'blease' and Initial = 'k'
Found 316 hits with Last Name = 'blease' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50364378
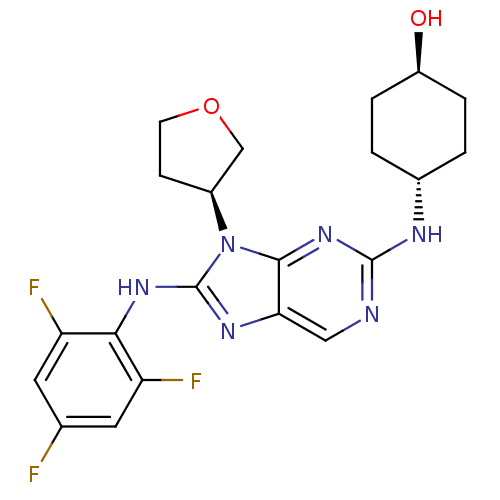
(CHEMBL1950289)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n([C@H]3CCOC3)c2n1 |r,wU:1.0,wD:4.7,25.26,(-6.26,-6.49,;-5.49,-5.16,;-6.25,-3.82,;-5.49,-2.5,;-3.95,-2.49,;-3.17,-3.82,;-3.94,-5.16,;-3.19,-1.16,;-1.65,-1.15,;-.89,.17,;.66,.18,;1.42,-1.15,;2.92,-1.46,;3.09,-2.98,;4.42,-3.75,;5.75,-2.97,;5.74,-1.45,;4.4,-.69,;7.06,-.67,;8.41,-1.43,;9.74,-.66,;8.41,-2.97,;7.08,-3.75,;7.08,-5.29,;1.69,-3.61,;1.23,-5.08,;-.23,-5.56,;-.23,-7.1,;1.24,-7.57,;2.14,-6.32,;.66,-2.48,;-.88,-2.48,)| Show InChI InChI=1S/C21H23F3N6O2/c22-11-7-15(23)18(16(24)8-11)28-21-27-17-9-25-20(26-12-1-3-14(31)4-2-12)29-19(17)30(21)13-5-6-32-10-13/h7-9,12-14,31H,1-6,10H2,(H,27,28)(H,25,26,29)/t12-,13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of hexa-His-tagged JNK2 expressed in baculoviral system using GST-tagged cJun as substrate preincubated for 15 mins prior ATP addition mea... |
Bioorg Med Chem Lett 22: 1433-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.027
BindingDB Entry DOI: 10.7270/Q2C829SK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50364378

(CHEMBL1950289)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n([C@H]3CCOC3)c2n1 |r,wU:1.0,wD:4.7,25.26,(-6.26,-6.49,;-5.49,-5.16,;-6.25,-3.82,;-5.49,-2.5,;-3.95,-2.49,;-3.17,-3.82,;-3.94,-5.16,;-3.19,-1.16,;-1.65,-1.15,;-.89,.17,;.66,.18,;1.42,-1.15,;2.92,-1.46,;3.09,-2.98,;4.42,-3.75,;5.75,-2.97,;5.74,-1.45,;4.4,-.69,;7.06,-.67,;8.41,-1.43,;9.74,-.66,;8.41,-2.97,;7.08,-3.75,;7.08,-5.29,;1.69,-3.61,;1.23,-5.08,;-.23,-5.56,;-.23,-7.1,;1.24,-7.57,;2.14,-6.32,;.66,-2.48,;-.88,-2.48,)| Show InChI InChI=1S/C21H23F3N6O2/c22-11-7-15(23)18(16(24)8-11)28-21-27-17-9-25-20(26-12-1-3-14(31)4-2-12)29-19(17)30(21)13-5-6-32-10-13/h7-9,12-14,31H,1-6,10H2,(H,27,28)(H,25,26,29)/t12-,13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of hexa-His-tagged JNK1 expressed in baculoviral system using GST-tagged c-Jun as substrate preincubated for 15 mins prior ATP addition me... |
Bioorg Med Chem Lett 22: 1433-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.027
BindingDB Entry DOI: 10.7270/Q2C829SK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM251460

(US9452998, 9)Show SMILES CC1(N)CCN(CC1)c1cccnc1NC(=O)c1nc(cnc1N)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C22H23F3N8O/c1-21(27)6-10-33(11-7-21)15-5-3-9-29-19(15)32-20(34)17-18(26)30-12-14(31-17)16-13(22(23,24)25)4-2-8-28-16/h2-5,8-9,12H,6-7,10-11,27H2,1H3,(H2,26,30)(H,29,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578357
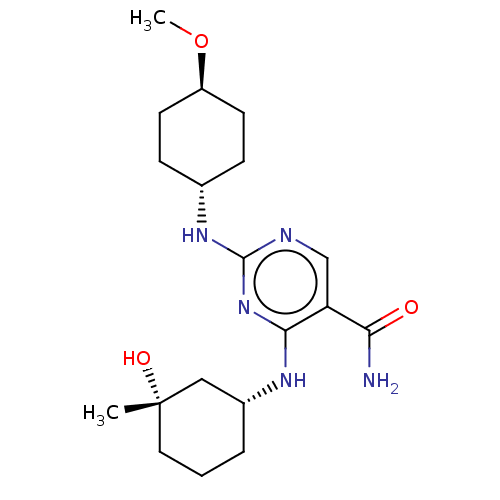
(CHEMBL4856984)Show SMILES CO[C@H]1CC[C@@H](CC1)Nc1ncc(C(N)=O)c(N[C@@H]2CCC[C@](C)(O)C2)n1 |r,wU:5.8,18.18,wD:22.23,2.1,(1.13,-3.13,;2.47,-2.37,;3.8,-3.14,;3.79,-4.69,;5.12,-5.47,;6.45,-4.69,;6.46,-3.15,;5.13,-2.38,;7.78,-5.46,;9.12,-4.69,;9.12,-3.15,;10.45,-2.38,;11.79,-3.14,;13.11,-2.37,;14.45,-3.13,;13.11,-.83,;11.79,-4.69,;13.13,-5.45,;13.14,-6.99,;11.81,-7.76,;11.81,-9.29,;13.15,-10.06,;14.47,-9.29,;15.24,-10.62,;16.01,-9.29,;14.48,-7.75,;10.45,-5.47,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578360
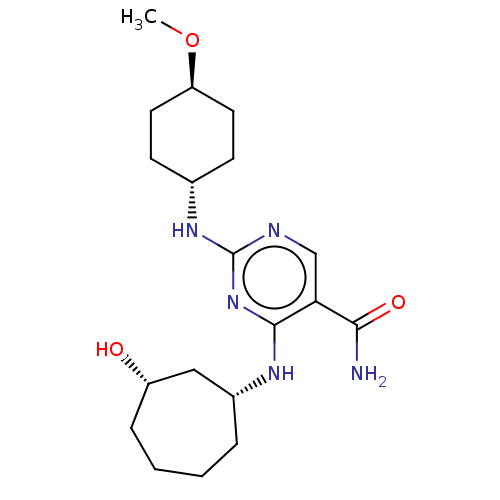
(CHEMBL4849353)Show SMILES CO[C@H]1CC[C@@H](CC1)Nc1ncc(C(N)=O)c(N[C@@H]2CCCC[C@H](O)C2)n1 |r,wU:5.8,18.18,23.24,wD:2.1,(62.95,-4.56,;64.29,-3.79,;65.62,-4.57,;65.61,-6.11,;66.95,-6.89,;68.27,-6.12,;68.28,-4.58,;66.96,-3.81,;69.61,-6.89,;70.94,-6.12,;70.94,-4.57,;72.27,-3.8,;73.61,-4.57,;74.94,-3.79,;76.27,-4.55,;74.93,-2.25,;73.62,-6.11,;74.95,-6.87,;74.96,-8.41,;73.53,-9.12,;73.24,-10.59,;74.17,-11.79,;75.64,-11.83,;76.67,-10.59,;78.16,-10.97,;76.35,-9.09,;72.27,-6.89,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573043

(CHEMBL4849510)Show SMILES CC1(C)[C@@H](O)C[C@H]1Nc1nc(NCc2cncnc2OCC(F)F)ncc1C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573038
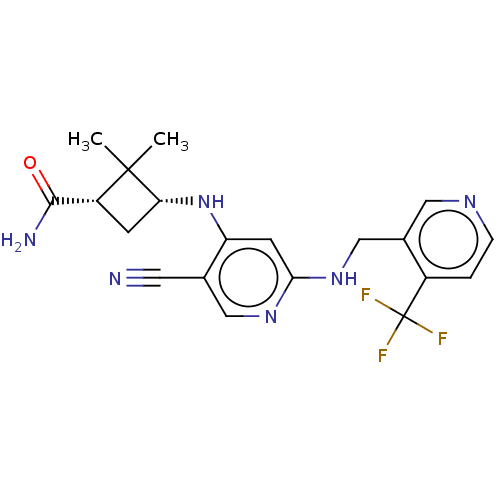
(CHEMBL4853353)Show SMILES CC1(C)[C@@H](C[C@@H]1C(N)=O)Nc1cc(NCc2cnccc2C(F)(F)F)ncc1C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM251460

(US9452998, 9)Show SMILES CC1(N)CCN(CC1)c1cccnc1NC(=O)c1nc(cnc1N)-c1ncccc1C(F)(F)F Show InChI InChI=1S/C22H23F3N8O/c1-21(27)6-10-33(11-7-21)15-5-3-9-29-19(15)32-20(34)17-18(26)30-12-14(31-17)16-13(22(23,24)25)4-2-8-28-16/h2-5,8-9,12H,6-7,10-11,27H2,1H3,(H2,26,30)(H,29,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKC-alpha (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578358
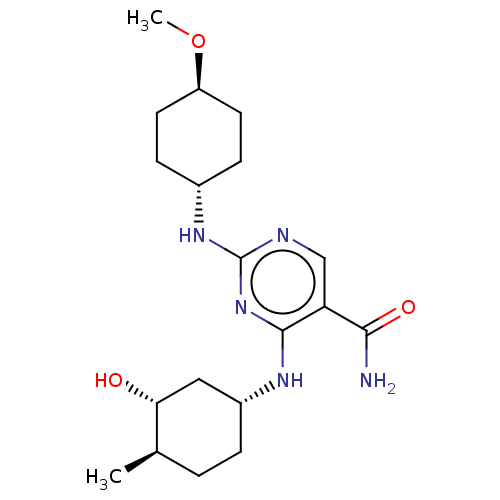
(CHEMBL4878370)Show SMILES CO[C@H]1CC[C@@H](CC1)Nc1ncc(C(N)=O)c(N[C@@H]2CC[C@@H](C)[C@H](O)C2)n1 |r,wU:5.8,18.18,23.24,wD:2.1,21.22,(19.9,-4.09,;21.24,-3.32,;22.57,-4.1,;22.57,-5.64,;23.9,-6.42,;25.23,-5.65,;25.24,-4.11,;23.91,-3.34,;26.56,-6.42,;27.89,-5.65,;27.9,-4.11,;29.22,-3.34,;30.56,-4.1,;31.89,-3.32,;33.23,-4.09,;31.88,-1.78,;30.57,-5.64,;31.91,-6.41,;31.91,-7.95,;30.58,-8.72,;30.59,-10.25,;31.92,-11.02,;31.92,-12.56,;33.25,-10.24,;34.58,-11.01,;33.25,-8.7,;29.23,-6.42,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578347
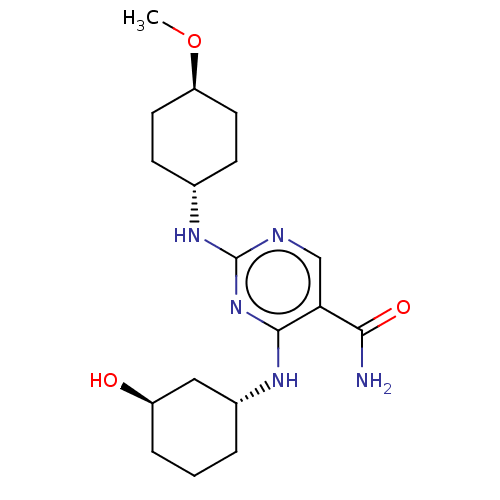
(CHEMBL4853125)Show SMILES CO[C@H]1CC[C@@H](CC1)Nc1ncc(C(N)=O)c(N[C@@H]2CCC[C@@H](O)C2)n1 |r,wU:5.8,18.18,wD:2.1,22.23,(41.41,-23.69,;42.75,-22.93,;44.08,-23.7,;44.07,-25.24,;45.41,-26.02,;46.73,-25.25,;46.74,-23.71,;45.42,-22.94,;48.07,-26.02,;49.4,-25.25,;49.4,-23.71,;50.73,-22.94,;52.07,-23.7,;53.4,-22.92,;54.73,-23.69,;53.39,-21.38,;52.08,-25.24,;53.41,-26.01,;53.42,-27.55,;52.09,-28.32,;52.09,-29.85,;53.43,-30.62,;54.76,-29.85,;56.1,-30.62,;54.76,-28.3,;50.73,-26.02,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578367
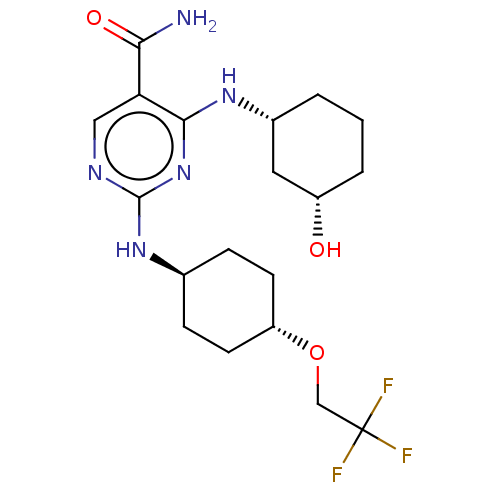
(CHEMBL4864618)Show SMILES NC(=O)c1cnc(N[C@H]2CC[C@@H](CC2)OCC(F)(F)F)nc1N[C@@H]1CCC[C@H](O)C1 |r,wU:8.7,23.24,27.29,wD:11.14,(79.91,-33.17,;78.57,-32.41,;78.56,-30.87,;77.24,-33.18,;75.9,-32.42,;74.57,-33.19,;74.57,-34.73,;73.24,-35.5,;71.9,-34.73,;70.58,-35.51,;69.24,-34.73,;69.25,-33.18,;70.59,-32.42,;71.91,-33.19,;67.92,-32.41,;66.58,-33.17,;65.25,-32.4,;65.26,-30.86,;63.91,-33.16,;63.91,-31.62,;75.91,-35.5,;77.25,-34.73,;78.58,-35.49,;78.59,-37.03,;77.26,-37.8,;77.27,-39.33,;78.6,-40.1,;79.93,-39.33,;81.26,-40.09,;79.93,-37.79,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573042

(CHEMBL4854104)Show SMILES CC(C)(F)c1ncncc1CNc1ncc(C#N)c(N[C@@H]2C[C@H](O)C2(C)C)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578365
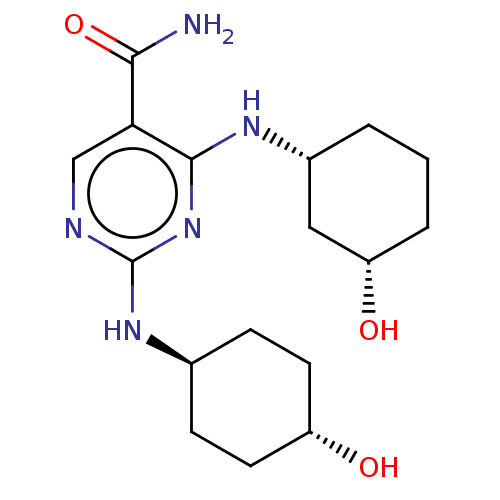
(CHEMBL4853525)Show SMILES NC(=O)c1cnc(N[C@H]2CC[C@H](O)CC2)nc1N[C@@H]1CCC[C@H](O)C1 |r,wU:8.7,18.19,22.24,wD:11.11,(15.33,-30.3,;13.99,-29.54,;13.99,-28,;12.66,-30.32,;11.33,-29.55,;10,-30.32,;10,-31.87,;8.66,-32.64,;7.33,-31.87,;6,-32.64,;4.67,-31.86,;4.67,-30.32,;3.34,-29.54,;6.01,-29.56,;7.34,-30.33,;11.33,-32.64,;12.67,-31.86,;14.01,-32.62,;14.02,-34.16,;12.69,-34.93,;12.69,-36.47,;14.02,-37.24,;15.35,-36.46,;16.69,-37.23,;15.36,-34.92,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578346
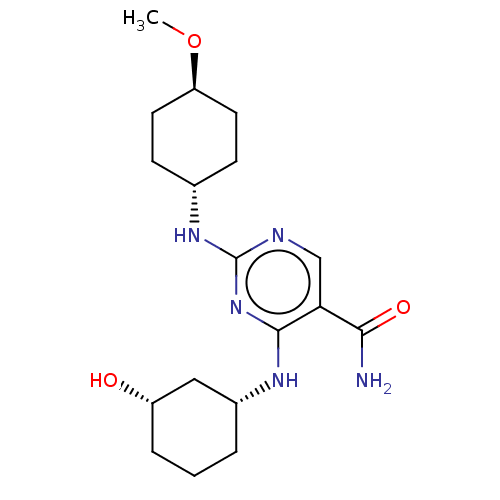
(CHEMBL4847078)Show SMILES CO[C@H]1CC[C@@H](CC1)Nc1ncc(C(N)=O)c(N[C@@H]2CCC[C@H](O)C2)n1 |r,wU:5.8,18.18,22.23,wD:2.1,(21,-22.65,;22.34,-21.88,;23.67,-22.66,;23.66,-24.2,;25,-24.98,;26.32,-24.21,;26.33,-22.67,;25.01,-21.9,;27.66,-24.98,;28.99,-24.21,;28.99,-22.66,;30.32,-21.89,;31.66,-22.66,;32.99,-21.88,;34.32,-22.64,;32.98,-20.34,;31.67,-24.2,;33,-24.96,;33.01,-26.5,;31.68,-27.27,;31.68,-28.81,;33.02,-29.58,;34.35,-28.8,;35.68,-29.57,;34.35,-27.26,;30.32,-24.98,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578366
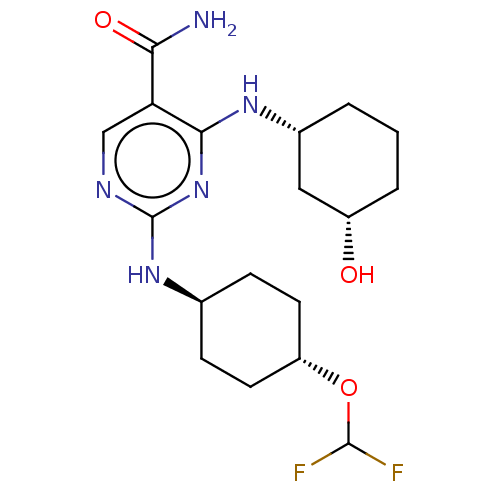
(CHEMBL4845965)Show SMILES NC(=O)c1cnc(N[C@H]2CC[C@@H](CC2)OC(F)F)nc1N[C@@H]1CCC[C@H](O)C1 |r,wU:8.7,21.22,25.27,wD:11.14,(57.85,-32.36,;56.51,-31.59,;56.51,-30.05,;55.18,-32.37,;53.85,-31.61,;52.52,-32.38,;52.52,-33.92,;51.18,-34.69,;49.85,-33.92,;48.52,-34.69,;47.19,-33.91,;47.19,-32.37,;48.53,-31.61,;49.86,-32.38,;45.86,-31.6,;44.53,-32.36,;43.2,-31.58,;44.52,-33.9,;53.85,-34.69,;55.19,-33.91,;56.53,-34.68,;56.54,-36.22,;55.21,-36.99,;55.21,-38.52,;56.54,-39.29,;57.87,-38.51,;59.21,-39.28,;57.88,-36.97,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578346

(CHEMBL4847078)Show SMILES CO[C@H]1CC[C@@H](CC1)Nc1ncc(C(N)=O)c(N[C@@H]2CCC[C@H](O)C2)n1 |r,wU:5.8,18.18,22.23,wD:2.1,(21,-22.65,;22.34,-21.88,;23.67,-22.66,;23.66,-24.2,;25,-24.98,;26.32,-24.21,;26.33,-22.67,;25.01,-21.9,;27.66,-24.98,;28.99,-24.21,;28.99,-22.66,;30.32,-21.89,;31.66,-22.66,;32.99,-21.88,;34.32,-22.64,;32.98,-20.34,;31.67,-24.2,;33,-24.96,;33.01,-26.5,;31.68,-27.27,;31.68,-28.81,;33.02,-29.58,;34.35,-28.8,;35.68,-29.57,;34.35,-27.26,;30.32,-24.98,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573034

(CHEMBL4859091)Show SMILES CC1(C)[C@@H](O)C[C@H]1Nc1nc(NCc2cnccc2C(F)(F)F)ncc1C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50572994

(CHEMBL4854326)Show SMILES CC1(C)CC(CC(C)(C)N1)Nc1cc(NCc2cnccc2C(F)(F)F)ncc1C#N | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578364
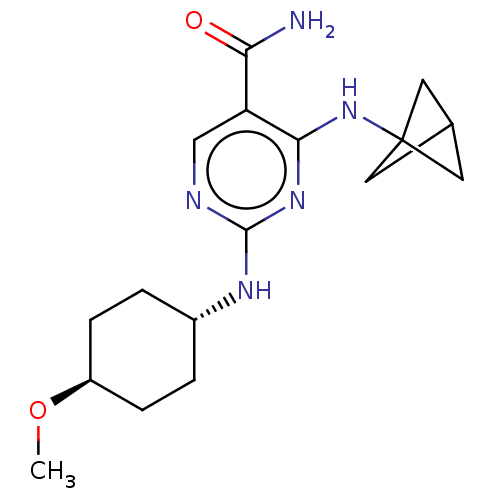
(CHEMBL4875131)Show SMILES CO[C@H]1CC[C@@H](CC1)Nc1ncc(C(N)=O)c(NC23CC(C2)C3)n1 |r,wU:5.8,wD:2.1,(64.75,-19.37,;66.09,-18.6,;67.42,-19.38,;67.42,-20.92,;68.75,-21.7,;70.08,-20.93,;70.09,-19.39,;68.76,-18.62,;71.41,-21.7,;72.75,-20.93,;72.75,-19.38,;74.08,-18.61,;75.41,-19.38,;76.74,-18.6,;78.08,-19.36,;76.73,-17.06,;75.42,-20.92,;76.76,-21.68,;76.76,-23.22,;75.68,-24.31,;76.78,-25.4,;77.86,-24.3,;76.29,-24.27,;74.08,-21.7,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50578361
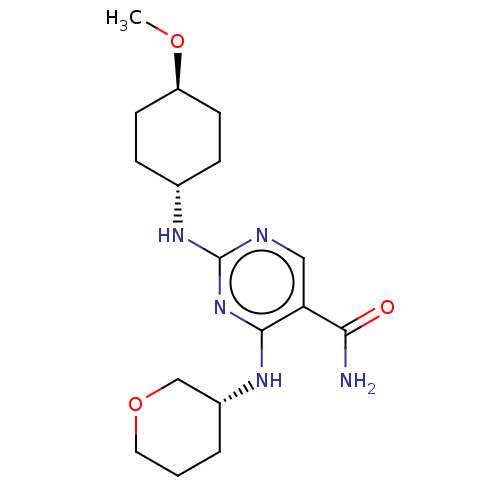
(CHEMBL4877560)Show SMILES CO[C@H]1CC[C@@H](CC1)Nc1ncc(C(N)=O)c(N[C@@H]2CCCOC2)n1 |r,wU:5.8,18.18,wD:2.1,(2.62,-17.13,;3.96,-16.37,;5.29,-17.15,;5.28,-18.69,;6.62,-19.47,;7.95,-18.69,;7.95,-17.15,;6.63,-16.38,;9.28,-19.46,;10.61,-18.7,;10.61,-17.15,;11.94,-16.38,;13.28,-17.14,;14.61,-16.37,;15.95,-17.13,;14.6,-14.83,;13.29,-18.69,;14.63,-19.45,;14.63,-20.99,;13.3,-21.76,;13.31,-23.29,;14.64,-24.07,;15.97,-23.29,;15.97,-21.75,;11.95,-19.47,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK2 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50578346

(CHEMBL4847078)Show SMILES CO[C@H]1CC[C@@H](CC1)Nc1ncc(C(N)=O)c(N[C@@H]2CCC[C@H](O)C2)n1 |r,wU:5.8,18.18,22.23,wD:2.1,(21,-22.65,;22.34,-21.88,;23.67,-22.66,;23.66,-24.2,;25,-24.98,;26.32,-24.21,;26.33,-22.67,;25.01,-21.9,;27.66,-24.98,;28.99,-24.21,;28.99,-22.66,;30.32,-21.89,;31.66,-22.66,;32.99,-21.88,;34.32,-22.64,;32.98,-20.34,;31.67,-24.2,;33,-24.96,;33.01,-26.5,;31.68,-27.27,;31.68,-28.81,;33.02,-29.58,;34.35,-28.8,;35.68,-29.57,;34.35,-27.26,;30.32,-24.98,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK2 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50364378

(CHEMBL1950289)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n([C@H]3CCOC3)c2n1 |r,wU:1.0,wD:4.7,25.26,(-6.26,-6.49,;-5.49,-5.16,;-6.25,-3.82,;-5.49,-2.5,;-3.95,-2.49,;-3.17,-3.82,;-3.94,-5.16,;-3.19,-1.16,;-1.65,-1.15,;-.89,.17,;.66,.18,;1.42,-1.15,;2.92,-1.46,;3.09,-2.98,;4.42,-3.75,;5.75,-2.97,;5.74,-1.45,;4.4,-.69,;7.06,-.67,;8.41,-1.43,;9.74,-.66,;8.41,-2.97,;7.08,-3.75,;7.08,-5.29,;1.69,-3.61,;1.23,-5.08,;-.23,-5.56,;-.23,-7.1,;1.24,-7.57,;2.14,-6.32,;.66,-2.48,;-.88,-2.48,)| Show InChI InChI=1S/C21H23F3N6O2/c22-11-7-15(23)18(16(24)8-11)28-21-27-17-9-25-20(26-12-1-3-14(31)4-2-12)29-19(17)30(21)13-5-6-32-10-13/h7-9,12-14,31H,1-6,10H2,(H,27,28)(H,25,26,29)/t12-,13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK2 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50578343
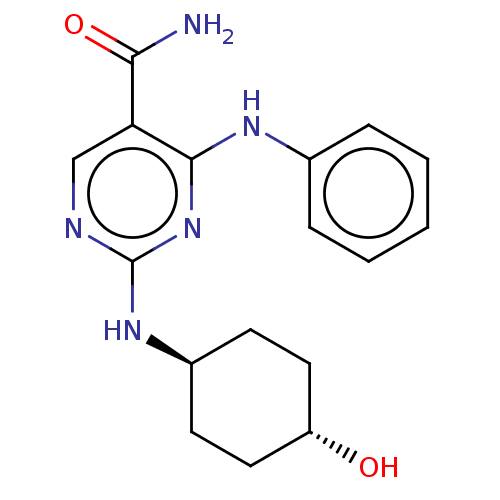
(CHEMBL4878046)Show SMILES NC(=O)c1cnc(N[C@H]2CC[C@H](O)CC2)nc1Nc1ccccc1 |r,wU:8.7,wD:11.11,(53.88,-7.89,;52.54,-7.13,;52.54,-5.59,;51.21,-7.9,;49.88,-7.14,;48.55,-7.91,;48.55,-9.45,;47.21,-10.22,;45.88,-9.45,;44.55,-10.23,;43.22,-9.45,;43.22,-7.9,;41.89,-7.13,;44.56,-7.14,;45.89,-7.91,;49.88,-10.23,;51.22,-9.45,;52.56,-10.21,;52.57,-11.75,;51.24,-12.52,;51.24,-14.06,;52.58,-14.82,;53.91,-14.04,;53.9,-12.5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK2 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573033
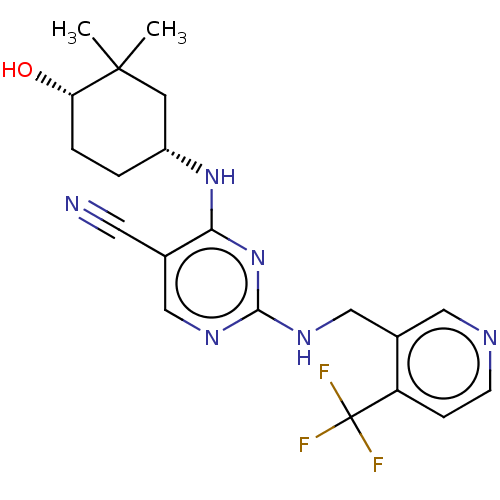
(CHEMBL4851695)Show SMILES CC1(C)C[C@@H](CC[C@@H]1O)Nc1nc(NCc2cnccc2C(F)(F)F)ncc1C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50364378

(CHEMBL1950289)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n([C@H]3CCOC3)c2n1 |r,wU:1.0,wD:4.7,25.26,(-6.26,-6.49,;-5.49,-5.16,;-6.25,-3.82,;-5.49,-2.5,;-3.95,-2.49,;-3.17,-3.82,;-3.94,-5.16,;-3.19,-1.16,;-1.65,-1.15,;-.89,.17,;.66,.18,;1.42,-1.15,;2.92,-1.46,;3.09,-2.98,;4.42,-3.75,;5.75,-2.97,;5.74,-1.45,;4.4,-.69,;7.06,-.67,;8.41,-1.43,;9.74,-.66,;8.41,-2.97,;7.08,-3.75,;7.08,-5.29,;1.69,-3.61,;1.23,-5.08,;-.23,-5.56,;-.23,-7.1,;1.24,-7.57,;2.14,-6.32,;.66,-2.48,;-.88,-2.48,)| Show InChI InChI=1S/C21H23F3N6O2/c22-11-7-15(23)18(16(24)8-11)28-21-27-17-9-25-20(26-12-1-3-14(31)4-2-12)29-19(17)30(21)13-5-6-32-10-13/h7-9,12-14,31H,1-6,10H2,(H,27,28)(H,25,26,29)/t12-,13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of hexa-His-tagged JNK3 expressed in baculoviral system using GST-tagged cJun as substrate preincubated for 15 mins prior ATP addition mea... |
Bioorg Med Chem Lett 22: 1433-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.027
BindingDB Entry DOI: 10.7270/Q2C829SK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50578347

(CHEMBL4853125)Show SMILES CO[C@H]1CC[C@@H](CC1)Nc1ncc(C(N)=O)c(N[C@@H]2CCC[C@@H](O)C2)n1 |r,wU:5.8,18.18,wD:2.1,22.23,(41.41,-23.69,;42.75,-22.93,;44.08,-23.7,;44.07,-25.24,;45.41,-26.02,;46.73,-25.25,;46.74,-23.71,;45.42,-22.94,;48.07,-26.02,;49.4,-25.25,;49.4,-23.71,;50.73,-22.94,;52.07,-23.7,;53.4,-22.92,;54.73,-23.69,;53.39,-21.38,;52.08,-25.24,;53.41,-26.01,;53.42,-27.55,;52.09,-28.32,;52.09,-29.85,;53.43,-30.62,;54.76,-29.85,;56.1,-30.62,;54.76,-28.3,;50.73,-26.02,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK2 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578343

(CHEMBL4878046)Show SMILES NC(=O)c1cnc(N[C@H]2CC[C@H](O)CC2)nc1Nc1ccccc1 |r,wU:8.7,wD:11.11,(53.88,-7.89,;52.54,-7.13,;52.54,-5.59,;51.21,-7.9,;49.88,-7.14,;48.55,-7.91,;48.55,-9.45,;47.21,-10.22,;45.88,-9.45,;44.55,-10.23,;43.22,-9.45,;43.22,-7.9,;41.89,-7.13,;44.56,-7.14,;45.89,-7.91,;49.88,-10.23,;51.22,-9.45,;52.56,-10.21,;52.57,-11.75,;51.24,-12.52,;51.24,-14.06,;52.58,-14.82,;53.91,-14.04,;53.9,-12.5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50364378

(CHEMBL1950289)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n([C@H]3CCOC3)c2n1 |r,wU:1.0,wD:4.7,25.26,(-6.26,-6.49,;-5.49,-5.16,;-6.25,-3.82,;-5.49,-2.5,;-3.95,-2.49,;-3.17,-3.82,;-3.94,-5.16,;-3.19,-1.16,;-1.65,-1.15,;-.89,.17,;.66,.18,;1.42,-1.15,;2.92,-1.46,;3.09,-2.98,;4.42,-3.75,;5.75,-2.97,;5.74,-1.45,;4.4,-.69,;7.06,-.67,;8.41,-1.43,;9.74,-.66,;8.41,-2.97,;7.08,-3.75,;7.08,-5.29,;1.69,-3.61,;1.23,-5.08,;-.23,-5.56,;-.23,-7.1,;1.24,-7.57,;2.14,-6.32,;.66,-2.48,;-.88,-2.48,)| Show InChI InChI=1S/C21H23F3N6O2/c22-11-7-15(23)18(16(24)8-11)28-21-27-17-9-25-20(26-12-1-3-14(31)4-2-12)29-19(17)30(21)13-5-6-32-10-13/h7-9,12-14,31H,1-6,10H2,(H,27,28)(H,25,26,29)/t12-,13-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of hexa-His-tagged JNK2 expressed in baculoviral system using GST-tagged cJun as substrate preincubated for 15 mins prior ATP addition mea... |
Bioorg Med Chem Lett 22: 1433-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.027
BindingDB Entry DOI: 10.7270/Q2C829SK |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573015
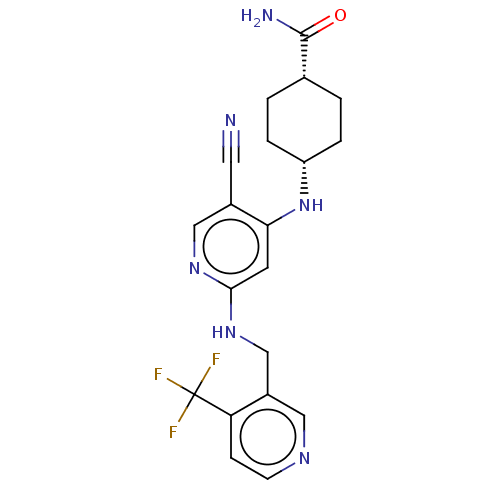
(CHEMBL4870774)Show SMILES NC(=O)[C@@H]1CC[C@@H](CC1)Nc1cc(NCc2cnccc2C(F)(F)F)ncc1C#N |r,wU:6.9,3.2,(38.42,-10.66,;37.11,-9.86,;37.14,-8.32,;35.76,-10.6,;34.44,-9.8,;33.08,-10.54,;33.06,-12.08,;34.37,-12.87,;35.72,-12.14,;31.71,-12.82,;31.68,-14.36,;30.33,-15.1,;30.31,-16.64,;28.96,-17.39,;28.93,-18.93,;27.58,-19.67,;27.56,-21.21,;26.21,-21.96,;24.89,-21.17,;24.92,-19.63,;26.27,-18.88,;26.29,-17.34,;24.75,-17.34,;25.52,-16,;27.64,-16.6,;31.62,-17.44,;32.97,-16.69,;33,-15.15,;34.35,-14.41,;35.69,-13.66,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50364377
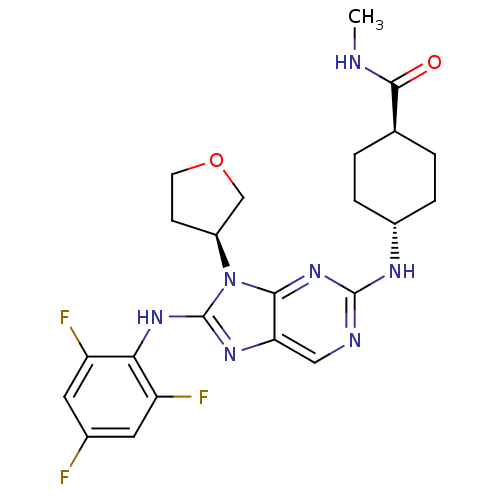
(CHEMBL1950304)Show SMILES CNC(=O)[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n([C@H]3CCOC3)c2n1 |r,wU:4.3,wD:28.29,7.10,(11.28,-17.55,;12.05,-16.21,;13.59,-16.22,;14.36,-17.55,;14.36,-14.88,;13.6,-13.55,;14.36,-12.22,;15.9,-12.22,;16.68,-13.54,;15.9,-14.88,;16.66,-10.88,;18.2,-10.88,;18.96,-9.55,;20.51,-9.54,;21.27,-10.87,;22.77,-11.19,;22.93,-12.71,;24.27,-13.47,;25.6,-12.7,;25.59,-11.17,;24.25,-10.41,;26.91,-10.39,;28.26,-11.16,;29.59,-10.38,;28.26,-12.7,;26.93,-13.47,;26.93,-15.01,;21.54,-13.33,;21.09,-14.81,;19.64,-15.31,;19.67,-16.85,;21.15,-17.3,;22.03,-16.04,;20.51,-12.2,;18.97,-12.2,)| Show InChI InChI=1S/C23H26F3N7O2/c1-27-21(34)12-2-4-14(5-3-12)29-22-28-10-18-20(32-22)33(15-6-7-35-11-15)23(30-18)31-19-16(25)8-13(24)9-17(19)26/h8-10,12,14-15H,2-7,11H2,1H3,(H,27,34)(H,30,31)(H,28,29,32)/t12-,14-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of hexa-His-tagged JNK3 expressed in baculoviral system using GST-tagged cJun as substrate preincubated for 15 mins prior ATP addition mea... |
Bioorg Med Chem Lett 22: 1433-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.027
BindingDB Entry DOI: 10.7270/Q2C829SK |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573029
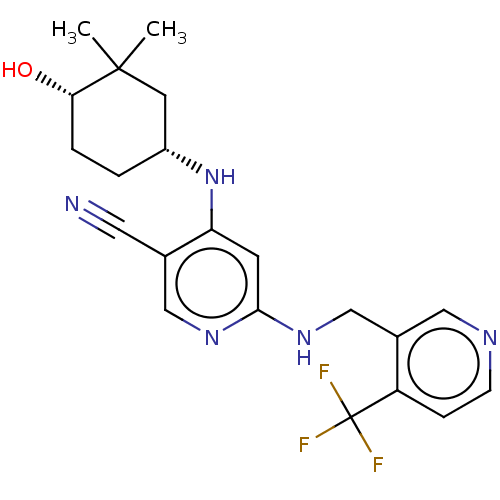
(CHEMBL4861883)Show SMILES CC1(C)C[C@@H](CC[C@@H]1O)Nc1cc(NCc2cnccc2C(F)(F)F)ncc1C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573044

(CHEMBL4873647)Show SMILES CC(F)(F)COc1ncncc1CNc1ncc(C#N)c(N[C@@H]2CC[C@H](O)C(C)(C)C2)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363465
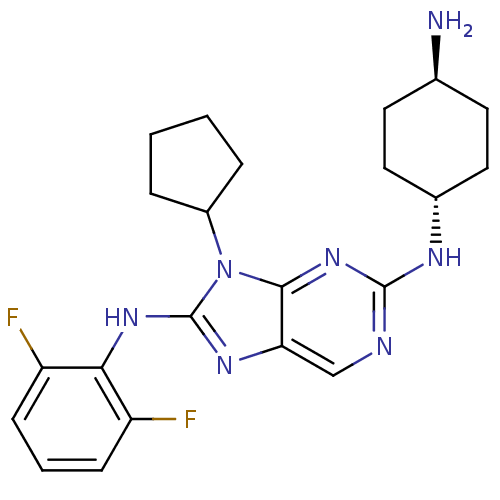
(CHEMBL1946646)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cccc3F)n(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(-8.05,-2.1,;-7.28,-.77,;-5.74,-.77,;-4.96,.56,;-5.74,1.89,;-7.27,1.89,;-8.04,.57,;-4.97,3.22,;-3.43,3.22,;-2.66,4.56,;-1.12,4.55,;-.36,3.22,;1.14,2.91,;1.29,1.38,;2.63,.61,;3.96,1.38,;3.96,2.92,;2.63,3.69,;5.29,3.7,;6.63,2.93,;6.63,1.38,;5.3,.61,;5.3,-.93,;-.11,.75,;-.11,-.78,;-1.36,-1.68,;-.89,-3.15,;.65,-3.15,;1.13,-1.69,;-1.14,1.9,;-2.66,1.9,)| Show InChI InChI=1S/C22H27F2N7/c23-16-6-3-7-17(24)19(16)29-22-28-18-12-26-21(27-14-10-8-13(25)9-11-14)30-20(18)31(22)15-4-1-2-5-15/h3,6-7,12-15H,1-2,4-5,8-11,25H2,(H,28,29)(H,26,27,30)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363455
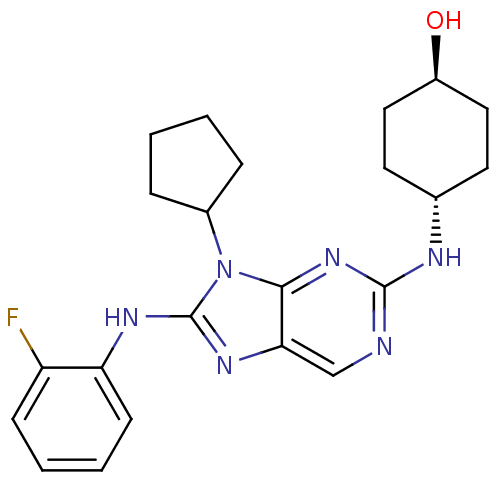
(CHEMBL1946333)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3ccccc3F)n(C3CCCC3)c2n1 |r,wU:1.0,wD:4.7,(-11.53,-21.51,;-10.75,-20.18,;-11.51,-18.84,;-10.74,-17.51,;-9.2,-17.52,;-8.43,-18.84,;-9.2,-20.18,;-8.44,-16.18,;-6.9,-16.18,;-6.13,-14.85,;-4.59,-14.85,;-3.83,-16.18,;-2.33,-16.49,;-2.17,-18.02,;-.84,-18.79,;.49,-18.03,;1.83,-18.8,;3.16,-18.02,;3.16,-16.47,;1.82,-15.71,;.5,-16.48,;-.84,-15.71,;-3.58,-18.65,;-3.58,-20.19,;-4.83,-21.09,;-4.36,-22.55,;-2.82,-22.56,;-2.34,-21.1,;-4.6,-17.51,;-6.13,-17.51,)| Show InChI InChI=1S/C22H27FN6O/c23-17-7-3-4-8-18(17)26-22-27-19-13-24-21(25-14-9-11-16(30)12-10-14)28-20(19)29(22)15-5-1-2-6-15/h3-4,7-8,13-16,30H,1-2,5-6,9-12H2,(H,26,27)(H,24,25,28)/t14-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50364377

(CHEMBL1950304)Show SMILES CNC(=O)[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n([C@H]3CCOC3)c2n1 |r,wU:4.3,wD:28.29,7.10,(11.28,-17.55,;12.05,-16.21,;13.59,-16.22,;14.36,-17.55,;14.36,-14.88,;13.6,-13.55,;14.36,-12.22,;15.9,-12.22,;16.68,-13.54,;15.9,-14.88,;16.66,-10.88,;18.2,-10.88,;18.96,-9.55,;20.51,-9.54,;21.27,-10.87,;22.77,-11.19,;22.93,-12.71,;24.27,-13.47,;25.6,-12.7,;25.59,-11.17,;24.25,-10.41,;26.91,-10.39,;28.26,-11.16,;29.59,-10.38,;28.26,-12.7,;26.93,-13.47,;26.93,-15.01,;21.54,-13.33,;21.09,-14.81,;19.64,-15.31,;19.67,-16.85,;21.15,-17.3,;22.03,-16.04,;20.51,-12.2,;18.97,-12.2,)| Show InChI InChI=1S/C23H26F3N7O2/c1-27-21(34)12-2-4-14(5-3-12)29-22-28-10-18-20(32-22)33(15-6-7-35-11-15)23(30-18)31-19-16(25)8-13(24)9-17(19)26/h8-10,12,14-15H,2-7,11H2,1H3,(H,27,34)(H,30,31)(H,28,29,32)/t12-,14-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of hexa-His-tagged JNK2 expressed in baculoviral system using GST-tagged cJun as substrate preincubated for 15 mins prior ATP addition mea... |
Bioorg Med Chem Lett 22: 1433-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.027
BindingDB Entry DOI: 10.7270/Q2C829SK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578352
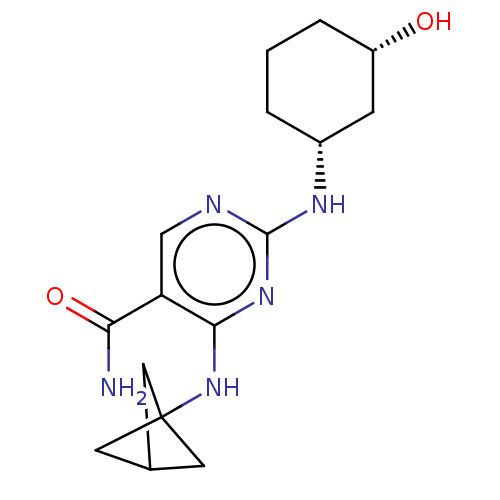
(CHEMBL4877655)Show SMILES NC(=O)c1cnc(N[C@@H]2CCC[C@H](O)C2)nc1NC12CC(C1)C2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578371
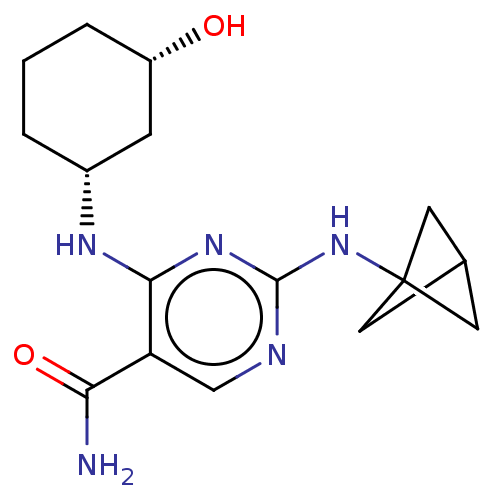
(CHEMBL4877389)Show SMILES NC(=O)c1cnc(NC23CC(C2)C3)nc1N[C@@H]1CCC[C@H](O)C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578351

(CHEMBL4862452)Show SMILES NC(=O)c1cnc(N[C@H]2CC[C@H](O)CC2)nc1NC12CC(C1)C2 |r,wU:8.7,wD:11.11,(35.38,-6.44,;34.05,-5.67,;34.04,-4.13,;32.72,-6.45,;31.38,-5.69,;30.05,-6.46,;30.05,-8,;28.72,-8.77,;27.38,-8,;26.06,-8.77,;24.72,-7.99,;24.73,-6.45,;23.4,-5.67,;26.07,-5.69,;27.39,-6.46,;31.38,-8.77,;32.73,-7.99,;34.06,-8.76,;34.07,-10.3,;32.99,-11.38,;34.08,-12.47,;35.17,-11.38,;33.65,-11.15,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578353
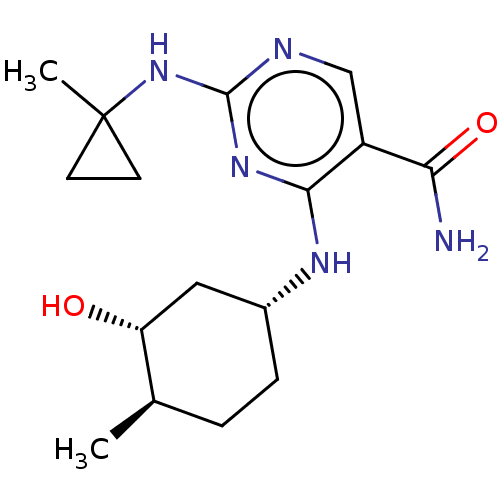
(CHEMBL4872066)Show SMILES C[C@@H]1CC[C@H](C[C@H]1O)Nc1nc(NC2(C)CC2)ncc1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363466
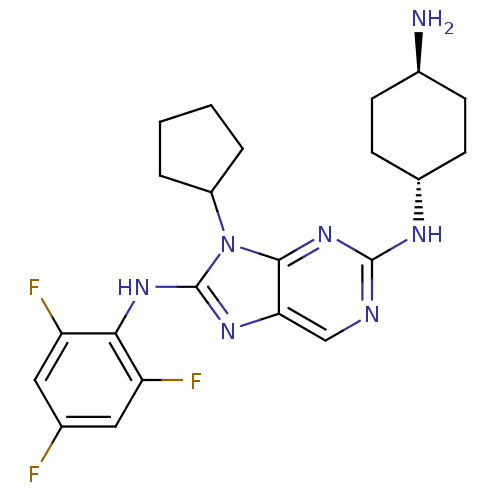
(CHEMBL1946647)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(9.91,-2.33,;10.68,-1,;12.22,-1.01,;13,.33,;12.22,1.65,;10.69,1.66,;9.92,.33,;12.99,2.99,;14.53,2.99,;15.3,4.32,;16.83,4.32,;17.6,2.99,;19.1,2.68,;19.25,1.15,;20.59,.38,;21.92,1.15,;21.92,2.69,;20.59,3.46,;23.25,3.46,;24.59,2.7,;25.92,3.47,;24.59,1.15,;23.26,.38,;23.25,-1.16,;17.85,.52,;17.85,-1.02,;16.6,-1.92,;17.07,-3.38,;18.61,-3.39,;19.09,-1.93,;16.82,1.67,;15.3,1.67,)| Show InChI InChI=1S/C22H26F3N7/c23-12-9-16(24)19(17(25)10-12)30-22-29-18-11-27-21(28-14-7-5-13(26)6-8-14)31-20(18)32(22)15-3-1-2-4-15/h9-11,13-15H,1-8,26H2,(H,29,30)(H,27,28,31)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578354
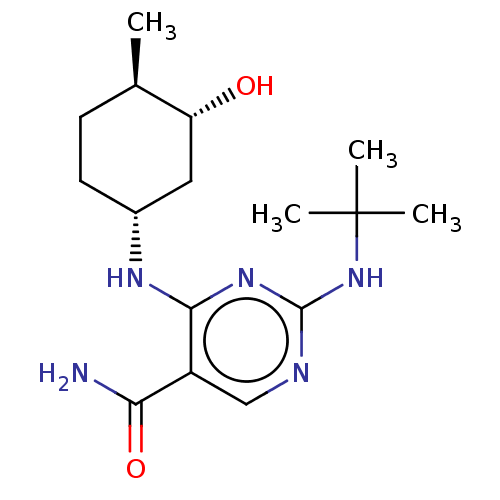
(CHEMBL4847106)Show SMILES C[C@@H]1CC[C@H](C[C@H]1O)Nc1nc(NC(C)(C)C)ncc1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363455

(CHEMBL1946333)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3ccccc3F)n(C3CCCC3)c2n1 |r,wU:1.0,wD:4.7,(-11.53,-21.51,;-10.75,-20.18,;-11.51,-18.84,;-10.74,-17.51,;-9.2,-17.52,;-8.43,-18.84,;-9.2,-20.18,;-8.44,-16.18,;-6.9,-16.18,;-6.13,-14.85,;-4.59,-14.85,;-3.83,-16.18,;-2.33,-16.49,;-2.17,-18.02,;-.84,-18.79,;.49,-18.03,;1.83,-18.8,;3.16,-18.02,;3.16,-16.47,;1.82,-15.71,;.5,-16.48,;-.84,-15.71,;-3.58,-18.65,;-3.58,-20.19,;-4.83,-21.09,;-4.36,-22.55,;-2.82,-22.56,;-2.34,-21.1,;-4.6,-17.51,;-6.13,-17.51,)| Show InChI InChI=1S/C22H27FN6O/c23-17-7-3-4-8-18(17)26-22-27-19-13-24-21(25-14-9-11-16(30)12-10-14)28-20(19)29(22)15-5-1-2-6-15/h3-4,7-8,13-16,30H,1-2,5-6,9-12H2,(H,26,27)(H,24,25,28)/t14-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50573027

(CHEMBL4867906)Show SMILES CC1(C)C[C@@H](CC(C)(C)[C@@H]1O)Nc1cc(NCc2cnccc2C(F)(F)F)ncc1C#N |r,wD:9.10,4.11,(16.5,-7.5,;15.98,-6.06,;17.5,-5.78,;14.65,-6.83,;13.31,-6.06,;13.31,-4.52,;14.65,-3.75,;15.63,-2.56,;13.65,-2.57,;15.98,-4.52,;17.31,-3.74,;11.98,-6.83,;11.98,-8.37,;10.65,-9.14,;10.65,-10.68,;9.32,-11.45,;9.32,-12.99,;7.98,-13.75,;7.98,-15.29,;6.64,-16.06,;5.32,-15.29,;5.32,-13.75,;6.64,-12.98,;6.64,-11.44,;5.15,-11.04,;6.25,-9.95,;7.98,-10.67,;11.98,-11.45,;13.32,-10.68,;13.32,-9.14,;14.65,-8.37,;15.98,-7.6,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PKA-theta (unknown origin) using Fam-labelled S6-derived peptide incubated for 2 hrs by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00388
BindingDB Entry DOI: 10.7270/Q22J6GPS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578350

(CHEMBL4874549)Show SMILES CC(C)(C)Nc1nc(N[C@H]2CC[C@H](O)CC2)ncc1C(N)=O |r,wU:9.8,wD:12.12,(14.02,-12,;15.35,-11.23,;16.69,-11.99,;15.34,-12.76,;15.34,-9.69,;14,-8.93,;12.66,-9.7,;11.33,-8.93,;9.99,-9.7,;8.66,-8.93,;7.33,-9.7,;6,-8.93,;6,-7.38,;4.67,-6.61,;7.34,-6.62,;8.67,-7.39,;11.33,-7.39,;12.66,-6.62,;13.99,-7.38,;15.32,-6.61,;16.66,-7.37,;15.32,-5.07,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50578351

(CHEMBL4862452)Show SMILES NC(=O)c1cnc(N[C@H]2CC[C@H](O)CC2)nc1NC12CC(C1)C2 |r,wU:8.7,wD:11.11,(35.38,-6.44,;34.05,-5.67,;34.04,-4.13,;32.72,-6.45,;31.38,-5.69,;30.05,-6.46,;30.05,-8,;28.72,-8.77,;27.38,-8,;26.06,-8.77,;24.72,-7.99,;24.73,-6.45,;23.4,-5.67,;26.07,-5.69,;27.39,-6.46,;31.38,-8.77,;32.73,-7.99,;34.06,-8.76,;34.07,-10.3,;32.99,-11.38,;34.08,-12.47,;35.17,-11.38,;33.65,-11.15,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK2 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363465

(CHEMBL1946646)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cccc3F)n(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(-8.05,-2.1,;-7.28,-.77,;-5.74,-.77,;-4.96,.56,;-5.74,1.89,;-7.27,1.89,;-8.04,.57,;-4.97,3.22,;-3.43,3.22,;-2.66,4.56,;-1.12,4.55,;-.36,3.22,;1.14,2.91,;1.29,1.38,;2.63,.61,;3.96,1.38,;3.96,2.92,;2.63,3.69,;5.29,3.7,;6.63,2.93,;6.63,1.38,;5.3,.61,;5.3,-.93,;-.11,.75,;-.11,-.78,;-1.36,-1.68,;-.89,-3.15,;.65,-3.15,;1.13,-1.69,;-1.14,1.9,;-2.66,1.9,)| Show InChI InChI=1S/C22H27F2N7/c23-16-6-3-7-17(24)19(16)29-22-28-18-12-26-21(27-14-10-8-13(25)9-11-14)30-20(18)31(22)15-4-1-2-5-15/h3,6-7,12-15H,1-2,4-5,8-11,25H2,(H,28,29)(H,26,27,30)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50363462
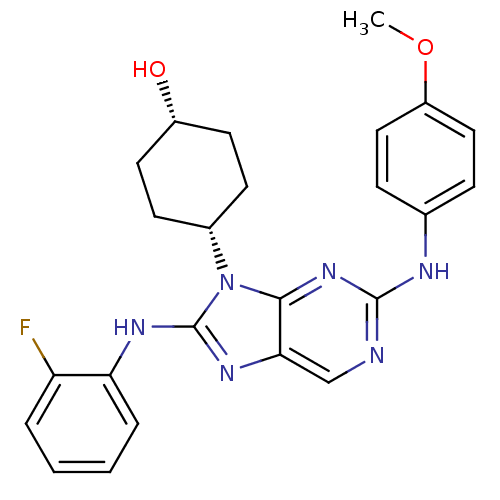
(CHEMBL1946340)Show SMILES COc1ccc(Nc2ncc3nc(Nc4ccccc4F)n([C@@H]4CC[C@H](O)CC4)c3n2)cc1 |r,wU:22.22,25.26,(4.81,-38.68,;6.35,-38.68,;7.12,-37.34,;6.35,-36.01,;7.12,-34.67,;8.66,-34.68,;9.43,-33.35,;10.97,-33.34,;11.74,-32.01,;13.27,-32.01,;14.04,-33.34,;15.54,-33.66,;15.7,-35.19,;17.03,-35.96,;18.37,-35.19,;19.7,-35.96,;21.04,-35.19,;21.04,-33.64,;19.7,-32.87,;18.37,-33.64,;17.03,-32.87,;14.29,-35.82,;14.29,-37.35,;12.95,-38.11,;12.94,-39.64,;14.27,-40.42,;14.26,-41.96,;15.61,-39.66,;15.62,-38.11,;13.26,-34.67,;11.74,-34.67,;9.43,-36,;8.67,-37.34,)| Show InChI InChI=1S/C24H25FN6O2/c1-33-18-12-6-15(7-13-18)27-23-26-14-21-22(30-23)31(16-8-10-17(32)11-9-16)24(29-21)28-20-5-3-2-4-19(20)25/h2-7,12-14,16-17,32H,8-11H2,1H3,(H,28,29)(H,26,27,30)/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50578369
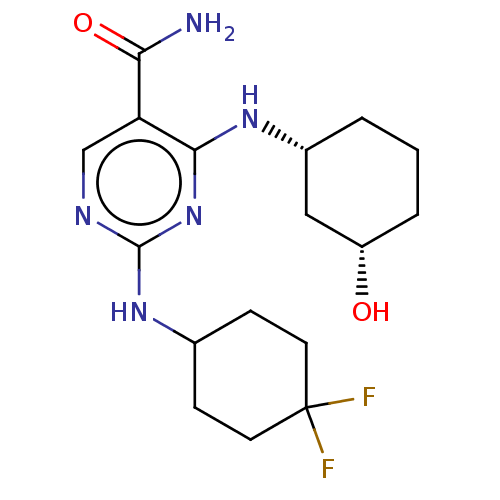
(CHEMBL4861847)Show SMILES NC(=O)c1cnc(NC2CCC(F)(F)CC2)nc1N[C@@H]1CCC[C@H](O)C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01716
BindingDB Entry DOI: 10.7270/Q22Z19CX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363466

(CHEMBL1946647)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1ncc2nc(Nc3c(F)cc(F)cc3F)n(C3CCCC3)c2n1 |r,wU:4.7,wD:1.0,(9.91,-2.33,;10.68,-1,;12.22,-1.01,;13,.33,;12.22,1.65,;10.69,1.66,;9.92,.33,;12.99,2.99,;14.53,2.99,;15.3,4.32,;16.83,4.32,;17.6,2.99,;19.1,2.68,;19.25,1.15,;20.59,.38,;21.92,1.15,;21.92,2.69,;20.59,3.46,;23.25,3.46,;24.59,2.7,;25.92,3.47,;24.59,1.15,;23.26,.38,;23.25,-1.16,;17.85,.52,;17.85,-1.02,;16.6,-1.92,;17.07,-3.38,;18.61,-3.39,;19.09,-1.93,;16.82,1.67,;15.3,1.67,)| Show InChI InChI=1S/C22H26F3N7/c23-12-9-16(24)19(17(25)10-12)30-22-29-18-11-27-21(28-14-7-5-13(26)6-8-14)31-20(18)32(22)15-3-1-2-4-15/h9-11,13-15H,1-8,26H2,(H,29,30)(H,27,28,31)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50363462

(CHEMBL1946340)Show SMILES COc1ccc(Nc2ncc3nc(Nc4ccccc4F)n([C@@H]4CC[C@H](O)CC4)c3n2)cc1 |r,wU:22.22,25.26,(4.81,-38.68,;6.35,-38.68,;7.12,-37.34,;6.35,-36.01,;7.12,-34.67,;8.66,-34.68,;9.43,-33.35,;10.97,-33.34,;11.74,-32.01,;13.27,-32.01,;14.04,-33.34,;15.54,-33.66,;15.7,-35.19,;17.03,-35.96,;18.37,-35.19,;19.7,-35.96,;21.04,-35.19,;21.04,-33.64,;19.7,-32.87,;18.37,-33.64,;17.03,-32.87,;14.29,-35.82,;14.29,-37.35,;12.95,-38.11,;12.94,-39.64,;14.27,-40.42,;14.26,-41.96,;15.61,-39.66,;15.62,-38.11,;13.26,-34.67,;11.74,-34.67,;9.43,-36,;8.67,-37.34,)| Show InChI InChI=1S/C24H25FN6O2/c1-33-18-12-6-15(7-13-18)27-23-26-14-21-22(30-23)31(16-8-10-17(32)11-9-16)24(29-21)28-20-5-3-2-4-19(20)25/h2-7,12-14,16-17,32H,8-11H2,1H3,(H,28,29)(H,26,27,30)/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting |
Bioorg Med Chem Lett 22: 1427-32 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.028
BindingDB Entry DOI: 10.7270/Q2513ZP6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data