 Found 232 hits with Last Name = 'hiesinger' and Initial = 'k'
Found 232 hits with Last Name = 'hiesinger' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50462118
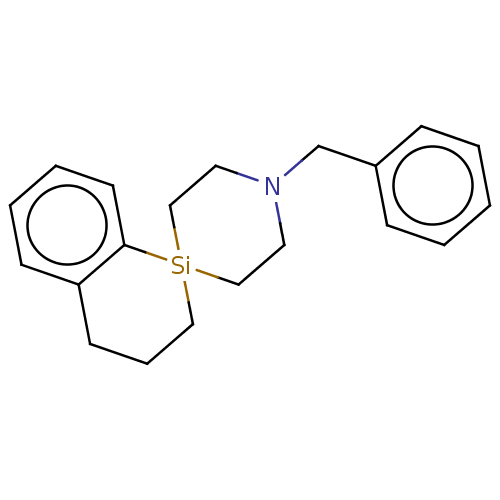
(CHEMBL4243296)Show SMILES [#6](-[#7]-1-[#6]-[#6][Si;v4]2([#6]-[#6]-[#6]-c3ccccc23)[#6]-[#6]-1)-c1ccccc1 Show InChI InChI=1S/C20H25NSi/c1-2-7-18(8-3-1)17-21-12-15-22(16-13-21)14-6-10-19-9-4-5-11-20(19)22/h1-5,7-9,11H,6,10,12-17H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01473
BindingDB Entry DOI: 10.7270/Q2QN6BRZ |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50035097
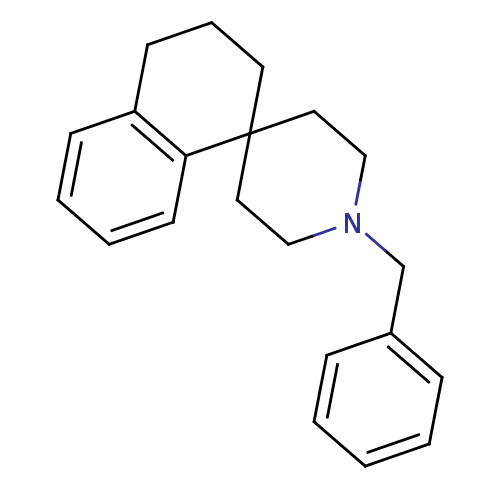
(1''-benzyl-3,4-dihydro-2H-spiro[naphthalene-1,4''-...)Show InChI InChI=1S/C21H25N/c1-2-7-18(8-3-1)17-22-15-13-21(14-16-22)12-6-10-19-9-4-5-11-20(19)21/h1-5,7-9,11H,6,10,12-17H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01473
BindingDB Entry DOI: 10.7270/Q2QN6BRZ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594427

(CHEMBL5172496)Show SMILES OC(=O)CCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:16:17:20:24.23.22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50035097

(1''-benzyl-3,4-dihydro-2H-spiro[naphthalene-1,4''-...)Show InChI InChI=1S/C21H25N/c1-2-7-18(8-3-1)17-22-15-13-21(14-16-22)12-6-10-19-9-4-5-11-20(19)21/h1-5,7-9,11H,6,10,12-17H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01473
BindingDB Entry DOI: 10.7270/Q2QN6BRZ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50327809
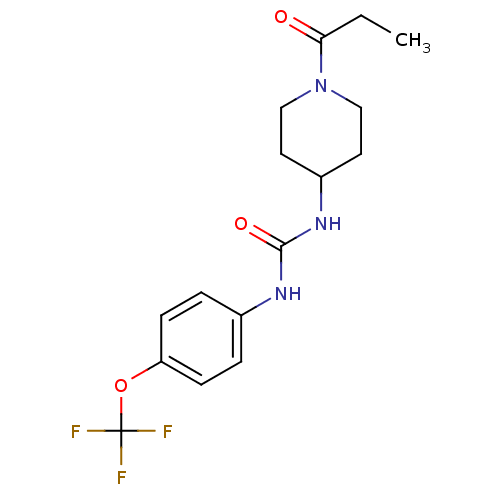
(1-(1-Propionylpiperidin-4-yl)-3-(4-(trifluorometho...)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C16H20F3N3O3/c1-2-14(23)22-9-7-12(8-10-22)21-15(24)20-11-3-5-13(6-4-11)25-16(17,18)19/h3-6,12H,2,7-10H2,1H3,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50502344

(Talinolol)Show InChI InChI=1S/C20H33N3O3/c1-20(2,3)21-13-17(24)14-26-18-11-9-16(10-12-18)23-19(25)22-15-7-5-4-6-8-15/h9-12,15,17,21,24H,4-8,13-14H2,1-3H3,(H2,22,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University of Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to beta1 adrenergic receptor (unknown origin) by radioligand binding assay |
ACS Med Chem Lett 10: 899-903 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00075
BindingDB Entry DOI: 10.7270/Q2R214M7 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50462118

(CHEMBL4243296)Show SMILES [#6](-[#7]-1-[#6]-[#6][Si;v4]2([#6]-[#6]-[#6]-c3ccccc23)[#6]-[#6]-1)-c1ccccc1 Show InChI InChI=1S/C20H25NSi/c1-2-7-18(8-3-1)17-21-12-15-22(16-13-21)14-6-10-19-9-4-5-11-20(19)22/h1-5,7-9,11H,6,10,12-17H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01473
BindingDB Entry DOI: 10.7270/Q2QN6BRZ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594426
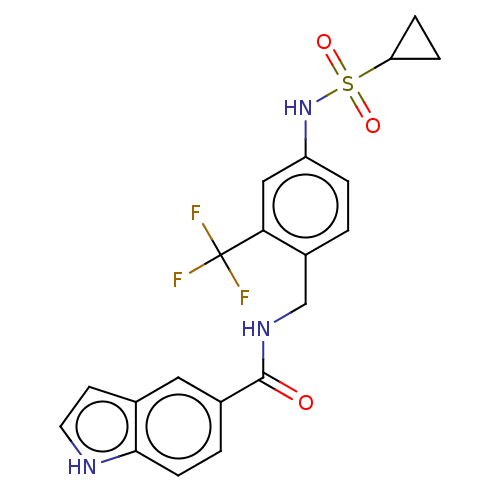
(CHEMBL5177249)Show SMILES FC(F)(F)c1cc(NS(=O)(=O)C2CC2)ccc1CNC(=O)c1ccc2[nH]ccc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50502344

(Talinolol)Show InChI InChI=1S/C20H33N3O3/c1-20(2,3)21-13-17(24)14-26-18-11-9-16(10-12-18)23-19(25)22-15-7-5-4-6-8-15/h9-12,15,17,21,24H,4-8,13-14H2,1-3H3,(H2,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University of Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to beta2 adrenergic receptor (unknown origin) by radioligand binding assay |
ACS Med Chem Lett 10: 899-903 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00075
BindingDB Entry DOI: 10.7270/Q2R214M7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50530480

(CHEMBL4445524)Show SMILES OC(=O)CCCc1nc(c(o1)-c1ccccc1)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H15Cl2NO3/c20-14-10-9-13(11-15(14)21)18-19(12-5-2-1-3-6-12)25-16(22-18)7-4-8-17(23)24/h1-3,5-6,9-11H,4,7-8H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50530480

(CHEMBL4445524)Show SMILES OC(=O)CCCc1nc(c(o1)-c1ccccc1)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H15Cl2NO3/c20-14-10-9-13(11-15(14)21)18-19(12-5-2-1-3-6-12)25-16(22-18)7-4-8-17(23)24/h1-3,5-6,9-11H,4,7-8H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50587703

(CHEMBL5174729)Show SMILES [H][C@]12CC[C@@]1([H])[C@@H](OC)\C=C\C[C@H](C)[C@@H](C)S(=O)(=O)NC(=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01473
BindingDB Entry DOI: 10.7270/Q2QN6BRZ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561492
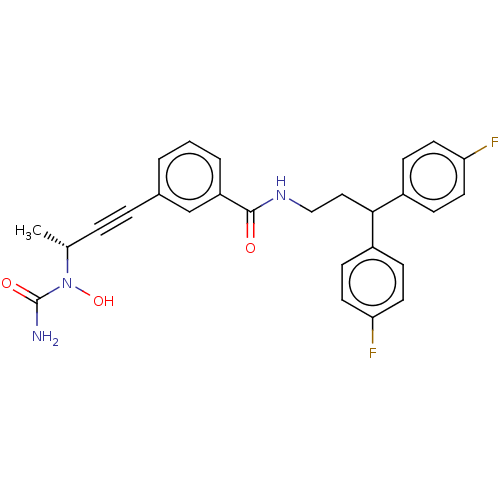
(CHEMBL4800490)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCC(c1ccc(F)cc1)c1ccc(F)cc1)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594428

(CHEMBL5192349)Show SMILES COc1ccc(CNC(=O)[C@@H]2CCC[C@@H](C2)Nc2ccc([N+]([O-])=O)c3nonc23)c(c1)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561490
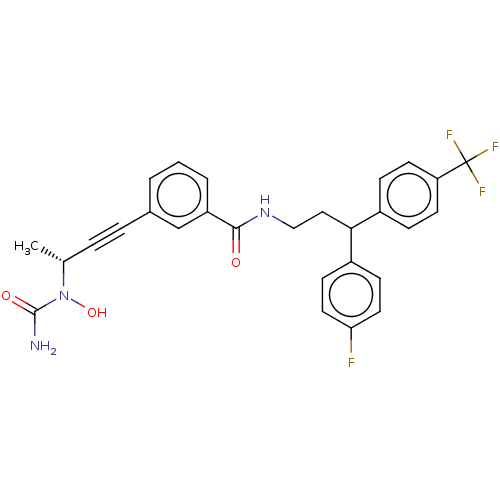
(CHEMBL4746942)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCC(c1ccc(F)cc1)c1ccc(cc1)C(F)(F)F)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561486

(CHEMBL4759111)Show SMILES CC(C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(Cl)cc1)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561482
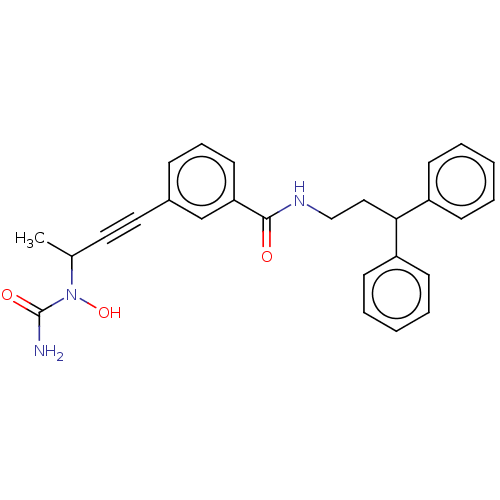
(CHEMBL4764099)Show SMILES CC(C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccccc1)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561483

(CHEMBL4795110)Show SMILES CC(C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(F)cc1)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561489

(CHEMBL4745687)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(Cl)c(Cl)c1)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561488

(CHEMBL4745452)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(cc1)S(N)(=O)=O)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561491
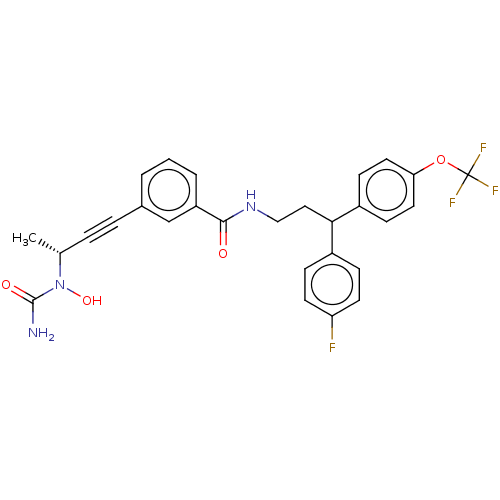
(CHEMBL4755533)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCC(c1ccc(F)cc1)c1ccc(OC(F)(F)F)cc1)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594427

(CHEMBL5172496)Show SMILES OC(=O)CCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:16:17:20:24.23.22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561484
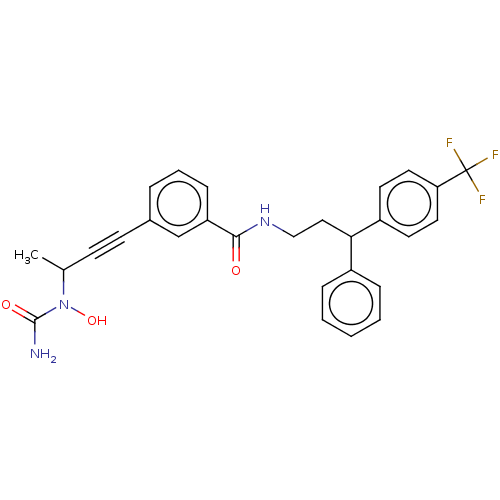
(CHEMBL4778283)Show SMILES CC(C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(cc1)C(F)(F)F)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561495
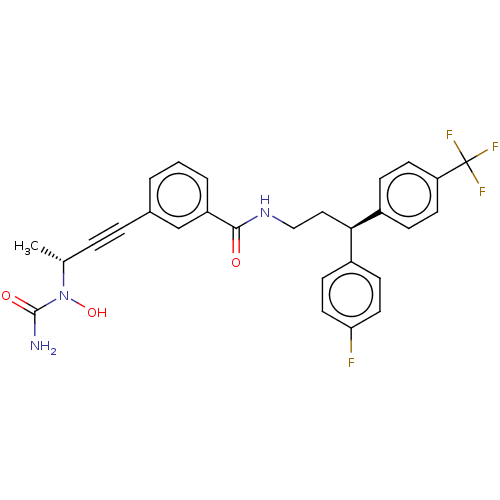
(CHEMBL4794423)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCC[C@H](c1ccc(F)cc1)c1ccc(cc1)C(F)(F)F)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561494
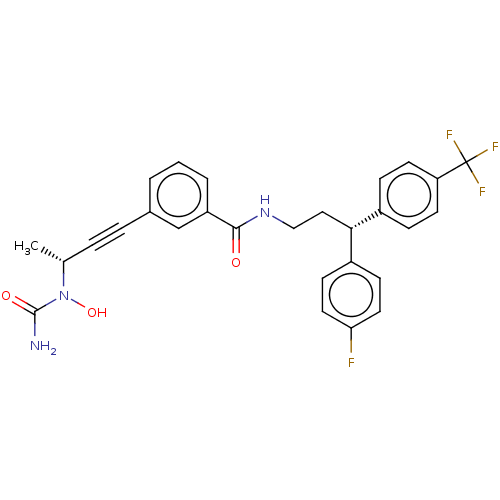
(CHEMBL4751593)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCC[C@@H](c1ccc(F)cc1)c1ccc(cc1)C(F)(F)F)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561487

(CHEMBL4759652)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(NS(C)(=O)=O)cc1)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561480
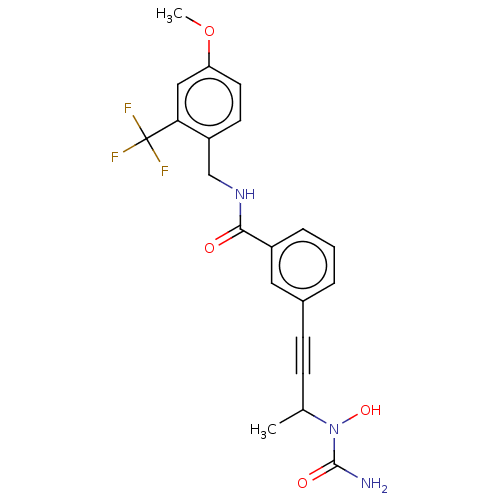
(CHEMBL4791222)Show SMILES COc1ccc(CNC(=O)c2cccc(c2)C#CC(C)N(O)C(N)=O)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561474
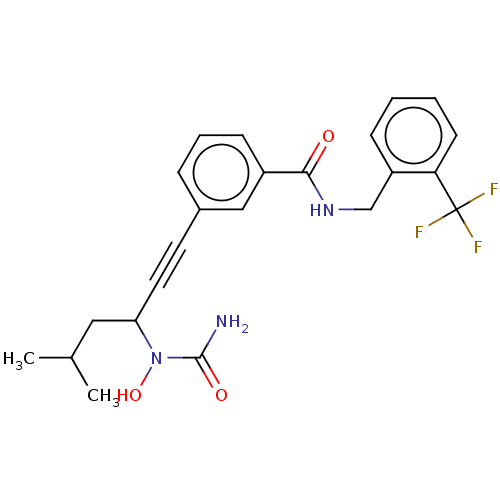
(CHEMBL4746544)Show SMILES CC(C)CC(C#Cc1cccc(c1)C(=O)NCc1ccccc1C(F)(F)F)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561493
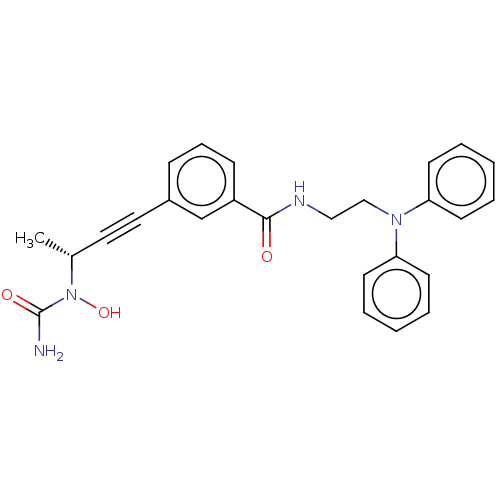
(CHEMBL4747688)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCN(c1ccccc1)c1ccccc1)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561485

(CHEMBL4757630)Show SMILES CC(C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(OC(F)(F)F)cc1)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50594428

(CHEMBL5192349)Show SMILES COc1ccc(CNC(=O)[C@@H]2CCC[C@@H](C2)Nc2ccc([N+]([O-])=O)c3nonc23)c(c1)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561473

(CHEMBL4753882)Show SMILES CC(C)C(C#Cc1cccc(c1)C(=O)NCc1ccccc1C(F)(F)F)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561472
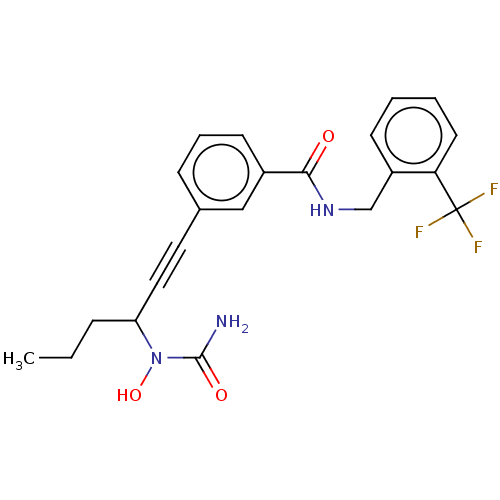
(CHEMBL4797528)Show SMILES CCCC(C#Cc1cccc(c1)C(=O)NCc1ccccc1C(F)(F)F)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50327809

(1-(1-Propionylpiperidin-4-yl)-3-(4-(trifluorometho...)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C16H20F3N3O3/c1-2-14(23)22-9-7-12(8-10-22)21-15(24)20-11-3-5-13(6-4-11)25-16(17,18)19/h3-6,12H,2,7-10H2,1H3,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50327809

(1-(1-Propionylpiperidin-4-yl)-3-(4-(trifluorometho...)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C16H20F3N3O3/c1-2-14(23)22-9-7-12(8-10-22)21-15(24)20-11-3-5-13(6-4-11)25-16(17,18)19/h3-6,12H,2,7-10H2,1H3,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length sEH (1 to 555 residues) expressed in Escherichia coli BL21 DE3 cells assessed as reduction in 6-methoxyna... |
ACS Med Chem Lett 11: 1244-1249 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00102
BindingDB Entry DOI: 10.7270/Q2FB56H6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561478
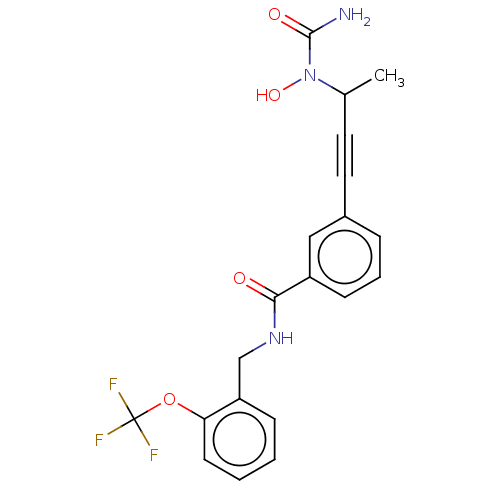
(CHEMBL4741953)Show SMILES CC(C#Cc1cccc(c1)C(=O)NCc1ccccc1OC(F)(F)F)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561471

(CHEMBL4781282)Show SMILES CCC(C#Cc1cccc(c1)C(=O)NCc1ccccc1C(F)(F)F)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594426

(CHEMBL5177249)Show SMILES FC(F)(F)c1cc(NS(=O)(=O)C2CC2)ccc1CNC(=O)c1ccc2[nH]ccc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561481
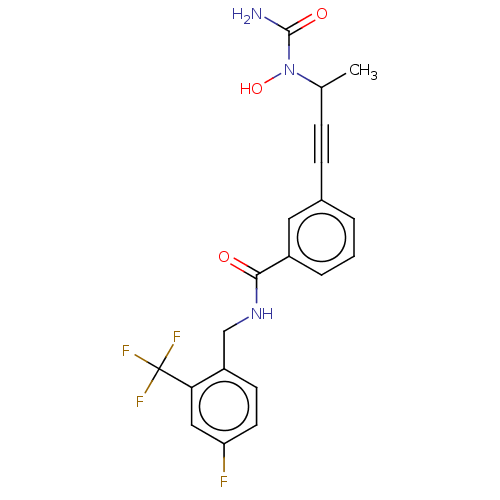
(CHEMBL4746074)Show SMILES CC(C#Cc1cccc(c1)C(=O)NCc1ccc(F)cc1C(F)(F)F)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594427

(CHEMBL5172496)Show SMILES OC(=O)CCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:16:17:20:24.23.22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561479
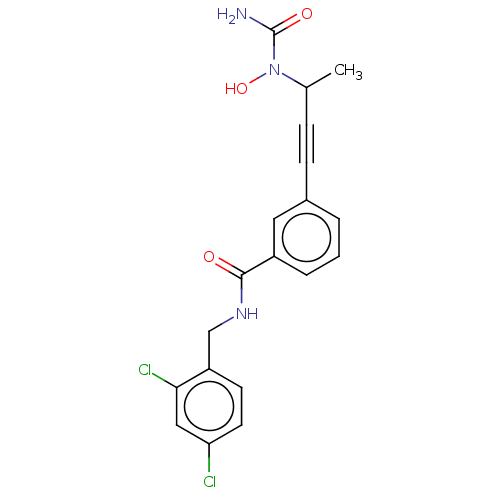
(CHEMBL4782144)Show SMILES CC(C#Cc1cccc(c1)C(=O)NCc1ccc(Cl)cc1Cl)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
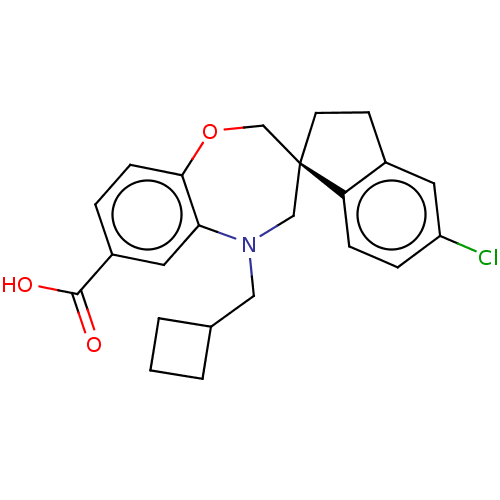
(Homo sapiens (Human)) | BDBM50519616
(CHEMBL4449176)Show SMILES OC(=O)c1ccc2OC[C@]3(CCc4cc(Cl)ccc34)CN(CC3CCC3)c2c1 |r| Show InChI InChI=1S/C23H24ClNO3/c24-18-5-6-19-16(10-18)8-9-23(19)13-25(12-15-2-1-3-15)20-11-17(22(26)27)4-7-21(20)28-14-23/h4-7,10-11,15H,1-3,8-9,12-14H2,(H,26,27)/t23-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01473
BindingDB Entry DOI: 10.7270/Q2QN6BRZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561468

(CHEMBL4745567)Show SMILES NC(=O)N(O)CC#Cc1cccc(c1)C(=O)NCc1ccccc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561469
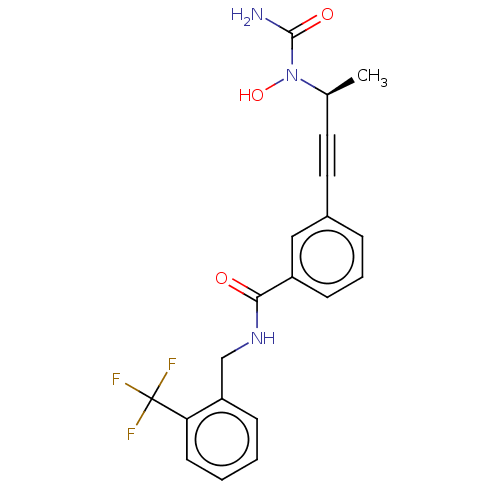
(CHEMBL4754077)Show SMILES C[C@@H](C#Cc1cccc(c1)C(=O)NCc1ccccc1C(F)(F)F)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561470
(CHEMBL4763763)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCc1ccccc1C(F)(F)F)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50502345

(CHEMBL4566250)Show SMILES CC(C)(C)N1CCOC(COc2ccc(NC(=O)NC3CCCCC3)cc2)C1 Show InChI InChI=1S/C22H35N3O3/c1-22(2,3)25-13-14-27-20(15-25)16-28-19-11-9-18(10-12-19)24-21(26)23-17-7-5-4-6-8-17/h9-12,17,20H,4-8,13-16H2,1-3H3,(H2,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University of Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length soluble epoxide hydrolase (1 to 555 residues) expressed in Escherichia coli BL21(DE3) using non-fluoresce... |
ACS Med Chem Lett 10: 899-903 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00075
BindingDB Entry DOI: 10.7270/Q2R214M7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data