

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566988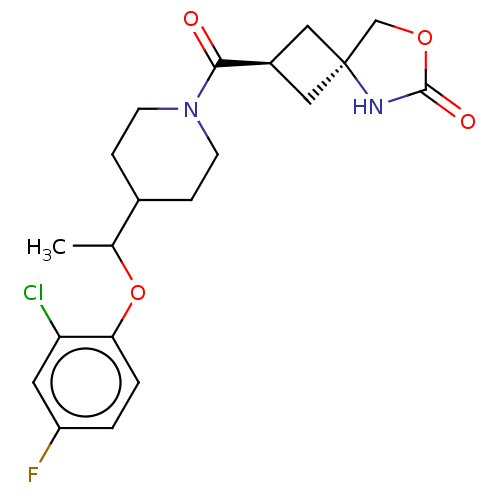 (CHEMBL4860900) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566987 (CHEMBL4854370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566989 (CHEMBL4872007) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566999 (CHEMBL4868762) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566984 (CHEMBL4870918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50567002 (CHEMBL4861633) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50567003 (CHEMBL4866307) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566990 (CHEMBL4871941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50567000 (CHEMBL4847556) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566998 (CHEMBL4865443) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50567005 (CHEMBL4845748) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50567001 (CHEMBL4873898) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50567004 (CHEMBL4854074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566982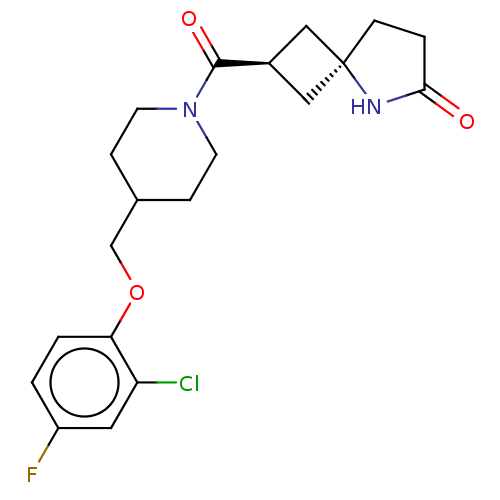 (CHEMBL4846513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566997 (CHEMBL4862009) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50567006 (CHEMBL4856861) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566991 (CHEMBL4872359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566981 (CHEMBL4850390) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Mus musculus (mouse)) | BDBM50567001 (CHEMBL4873898) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50585896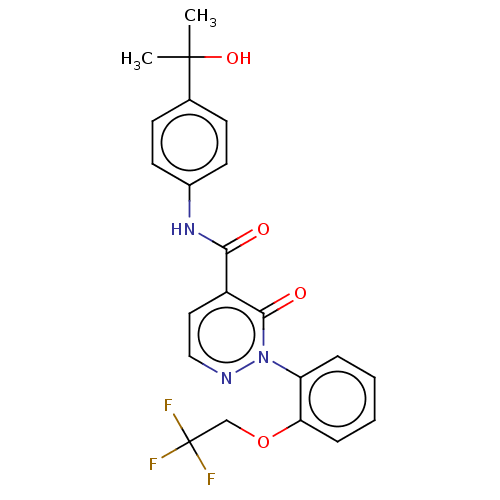 (CHEMBL5085509) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566972 (CHEMBL4870749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50585892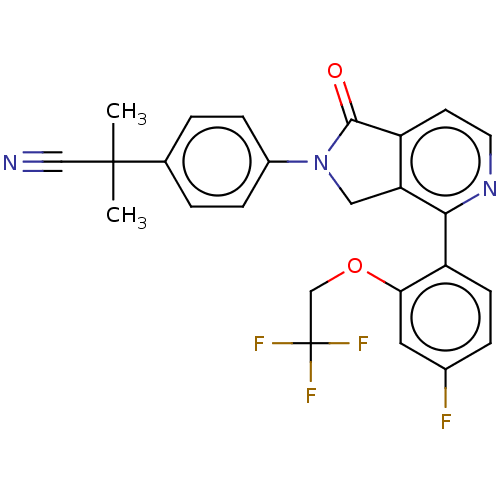 (CHEMBL5092141) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566996 (CHEMBL4847831) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50585900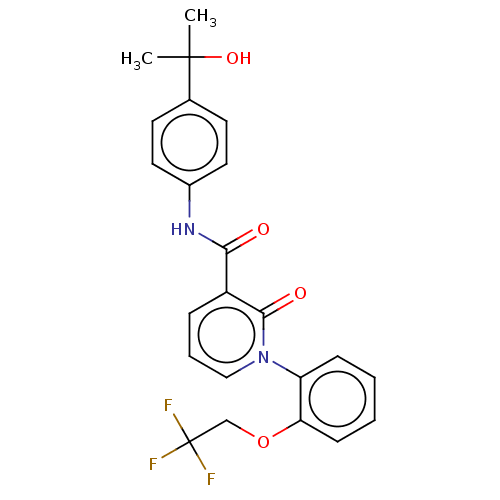 (CHEMBL5083428) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566973 (CHEMBL4872139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566974 (CHEMBL4855733) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566994 (CHEMBL4862447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50585893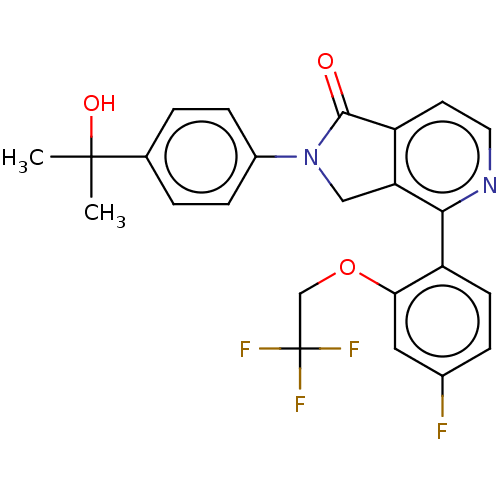 (CHEMBL5078078) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566975 (CHEMBL4867138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566995 (CHEMBL4870417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566993 (CHEMBL4874410) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566980 (CHEMBL4867695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50585894 (CHEMBL5077442) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50585899 (CHEMBL5086168) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50585897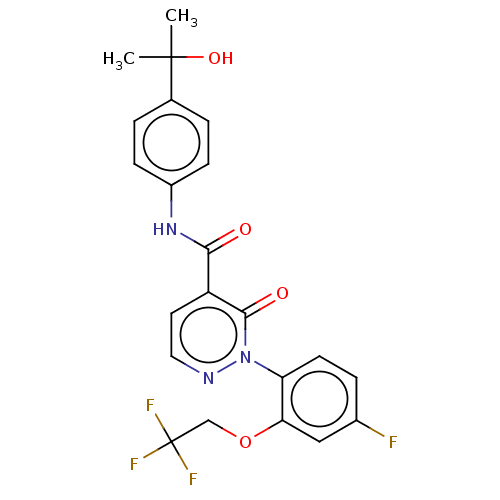 (CHEMBL5086523) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50585889 (CHEMBL5091974) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50585893 (CHEMBL5078078) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566992 (CHEMBL4854188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566977 (CHEMBL4849041) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566985 (CHEMBL4859927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50585886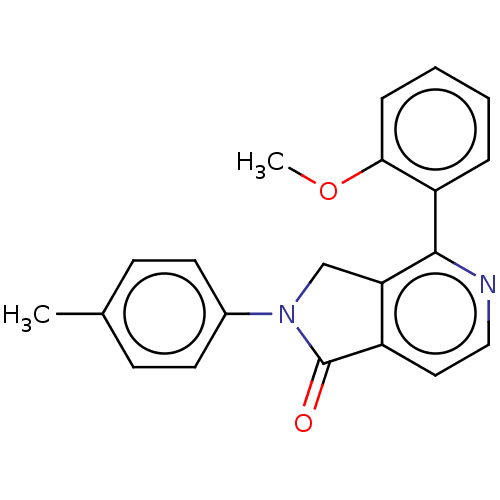 (CHEMBL5076612) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50585898 (CHEMBL5091003) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50585896 (CHEMBL5085509) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566978 (CHEMBL4870350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50585893 (CHEMBL5078078) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human SERT | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566979 (CHEMBL4876184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50585891 (CHEMBL5089984) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50585890 (CHEMBL5081829) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50566983 (CHEMBL4864582) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human His-tagged MAGL assessed as formation of arachidonic acid using 2-acylglycerol incubated for 10 mins by mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00432 BindingDB Entry DOI: 10.7270/Q2M90DDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50585887 (CHEMBL5088946) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GCS using C8-ceramide and UDP-glucose as substrate incubated for 1 hr by RapidFire mass spectrometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02078 BindingDB Entry DOI: 10.7270/Q2KW5KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 73 total ) | Next | Last >> |