

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50513186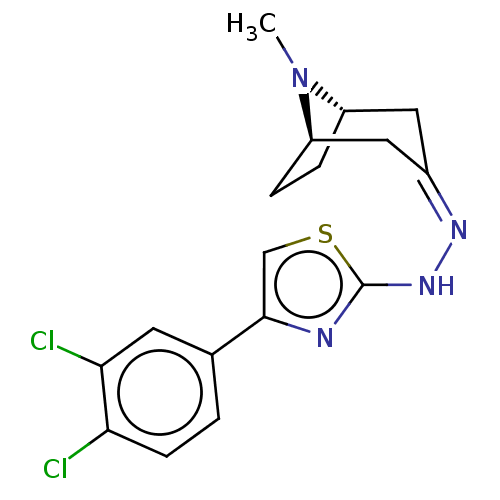 (CHEMBL4588381) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method | Eur J Med Chem 175: 162-171 (2019) Article DOI: 10.1016/j.ejmech.2019.05.006 BindingDB Entry DOI: 10.7270/Q2794815 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50513187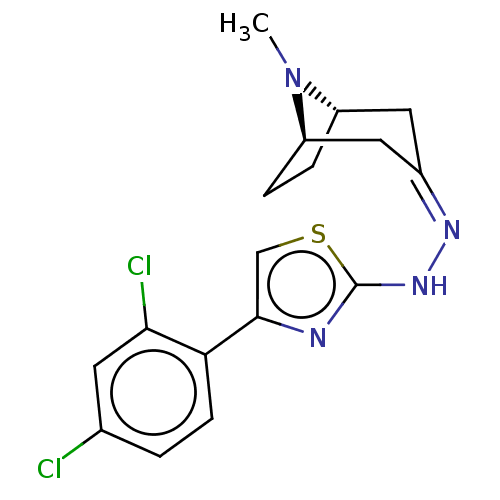 (CHEMBL4583240) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method | Eur J Med Chem 175: 162-171 (2019) Article DOI: 10.1016/j.ejmech.2019.05.006 BindingDB Entry DOI: 10.7270/Q2794815 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50513185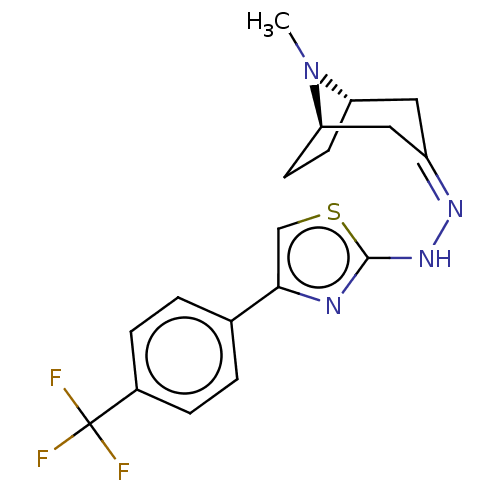 (CHEMBL4463818) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method | Eur J Med Chem 175: 162-171 (2019) Article DOI: 10.1016/j.ejmech.2019.05.006 BindingDB Entry DOI: 10.7270/Q2794815 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method | Eur J Med Chem 175: 162-171 (2019) Article DOI: 10.1016/j.ejmech.2019.05.006 BindingDB Entry DOI: 10.7270/Q2794815 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50513188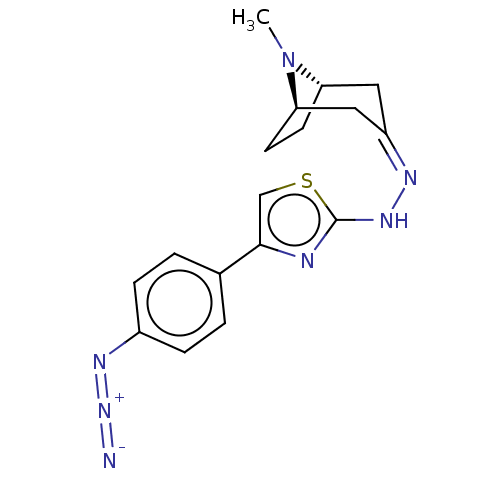 (CHEMBL4453112) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method | Eur J Med Chem 175: 162-171 (2019) Article DOI: 10.1016/j.ejmech.2019.05.006 BindingDB Entry DOI: 10.7270/Q2794815 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50513183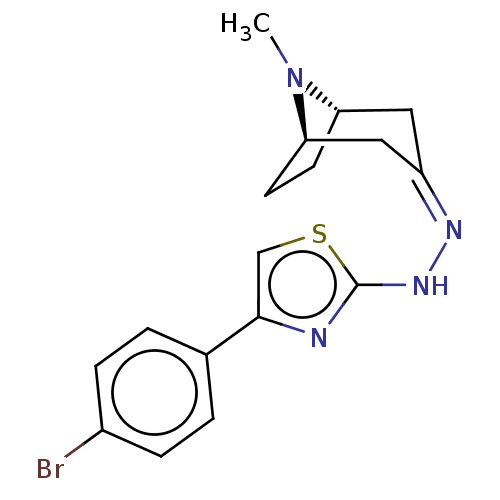 (CHEMBL4473661) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method | Eur J Med Chem 175: 162-171 (2019) Article DOI: 10.1016/j.ejmech.2019.05.006 BindingDB Entry DOI: 10.7270/Q2794815 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50513190 (CHEMBL4438867) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method | Eur J Med Chem 175: 162-171 (2019) Article DOI: 10.1016/j.ejmech.2019.05.006 BindingDB Entry DOI: 10.7270/Q2794815 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50513189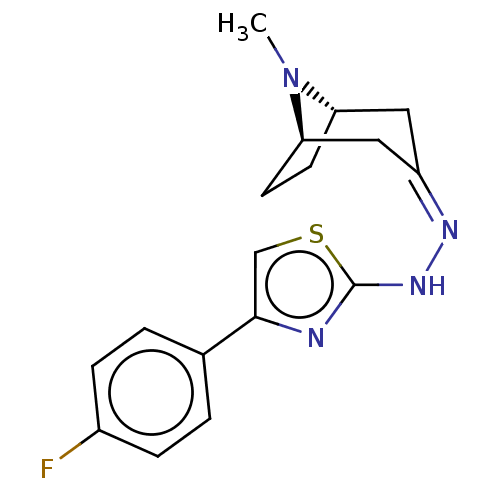 (CHEMBL4555931) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method | Eur J Med Chem 175: 162-171 (2019) Article DOI: 10.1016/j.ejmech.2019.05.006 BindingDB Entry DOI: 10.7270/Q2794815 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50513184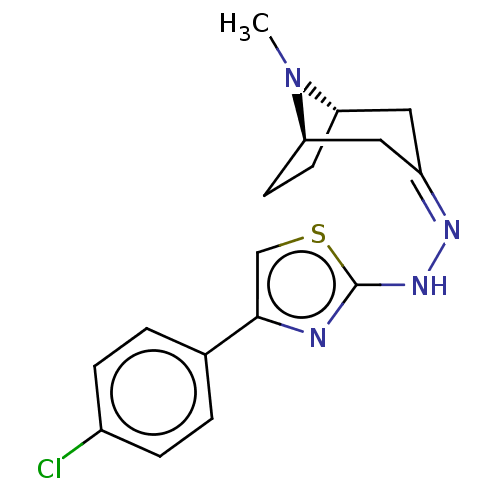 (CHEMBL4456453) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method | Eur J Med Chem 175: 162-171 (2019) Article DOI: 10.1016/j.ejmech.2019.05.006 BindingDB Entry DOI: 10.7270/Q2794815 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50351096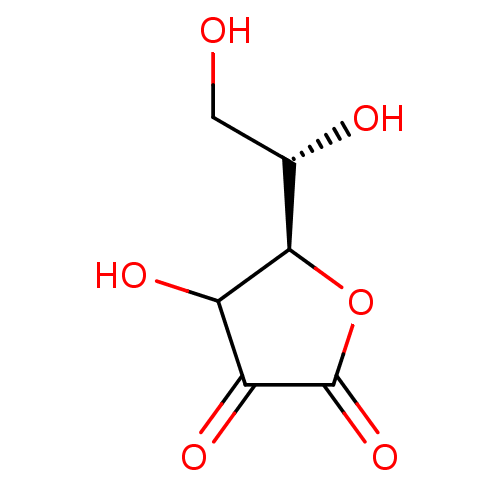 (ASCORBIC ACID) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method | Eur J Med Chem 175: 162-171 (2019) Article DOI: 10.1016/j.ejmech.2019.05.006 BindingDB Entry DOI: 10.7270/Q2794815 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||