 Found 223 hits with Last Name = 'shankaran' and Initial = 'k'
Found 223 hits with Last Name = 'shankaran' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50148285

(2-{(S)-3-[4-(2-Benzyl-thiazol-5-yl)-piperidin-1-yl...)Show SMILES OC(=O)C(CC1CCC1)N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)s2)C(C1)c1ccccc1 Show InChI InChI=1S/C33H41N3O2S/c37-33(38)30(18-24-10-7-11-24)36-22-28(29(23-36)26-12-5-2-6-13-26)21-35-16-14-27(15-17-35)31-20-34-32(39-31)19-25-8-3-1-4-9-25/h1-6,8-9,12-13,20,24,27-30H,7,10-11,14-19,21-23H2,(H,37,38)/t28-,29?,30?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50148286
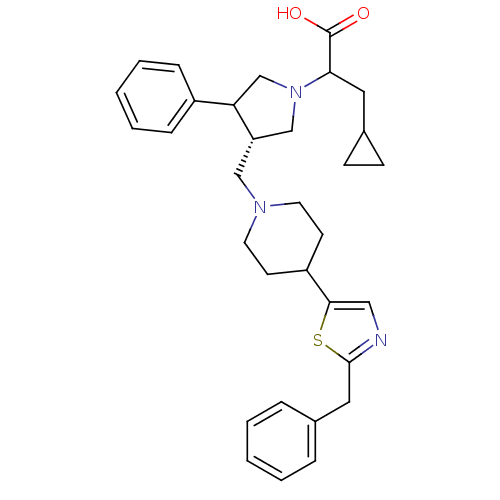
(2-{(S)-3-[4-(2-Benzyl-thiazol-5-yl)-piperidin-1-yl...)Show SMILES OC(=O)C(CC1CC1)N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)s2)C(C1)c1ccccc1 Show InChI InChI=1S/C32H39N3O2S/c36-32(37)29(17-24-11-12-24)35-21-27(28(22-35)25-9-5-2-6-10-25)20-34-15-13-26(14-16-34)30-19-33-31(38-30)18-23-7-3-1-4-8-23/h1-10,19,24,26-29H,11-18,20-22H2,(H,36,37)/t27-,28?,29?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249465

((R)-2-((3S,4S)-3-((4-(2-benzylthiazol-5-yl)piperid...)Show SMILES OC(=O)[C@@H](C1CCCCC1)N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)s2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C34H43N3O2S/c38-34(39)33(28-14-8-3-9-15-28)37-23-29(30(24-37)26-12-6-2-7-13-26)22-36-18-16-27(17-19-36)31-21-35-32(40-31)20-25-10-4-1-5-11-25/h1-2,4-7,10-13,21,27-30,33H,3,8-9,14-20,22-24H2,(H,38,39)/t29-,30+,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249467

((R)-2-((3S,4S)-3-((4-(2-benzyloxazol-5-yl)piperidi...)Show SMILES OC(=O)[C@@H](CC1CCC1)N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)o2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C33H41N3O3/c37-33(38)30(18-24-10-7-11-24)36-22-28(29(23-36)26-12-5-2-6-13-26)21-35-16-14-27(15-17-35)31-20-34-32(39-31)19-25-8-3-1-4-9-25/h1-6,8-9,12-13,20,24,27-30H,7,10-11,14-19,21-23H2,(H,37,38)/t28-,29+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50404275
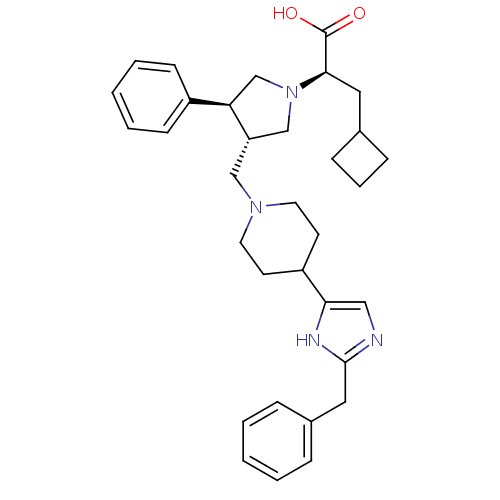
(CHEMBL2112559)Show SMILES OC(=O)[C@@H](CC1CCC1)N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)[nH]2)[C@H](C1)c1ccccc1 Show InChI InChI=1S/C33H42N4O2/c38-33(39)31(18-24-10-7-11-24)37-22-28(29(23-37)26-12-5-2-6-13-26)21-36-16-14-27(15-17-36)30-20-34-32(35-30)19-25-8-3-1-4-9-25/h1-6,8-9,12-13,20,24,27-29,31H,7,10-11,14-19,21-23H2,(H,34,35)(H,38,39)/t28-,29+,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249334
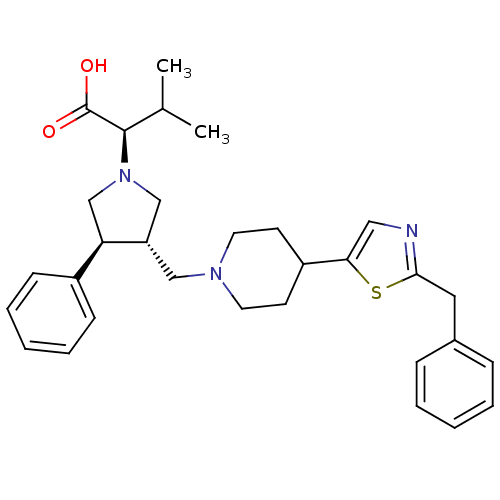
((R)-2-((3S,4S)-3-((4-(2-benzylthiazol-5-yl)piperid...)Show SMILES CC(C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)s2)[C@H](C1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C31H39N3O2S/c1-22(2)30(31(35)36)34-20-26(27(21-34)24-11-7-4-8-12-24)19-33-15-13-25(14-16-33)28-18-32-29(37-28)17-23-9-5-3-6-10-23/h3-12,18,22,25-27,30H,13-17,19-21H2,1-2H3,(H,35,36)/t26-,27+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50404276

(CHEMBL2112558)Show SMILES CCc1nc(Cc2ccccc2)[nH]c1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](CC2CCC2)C(O)=O)CC1 Show InChI InChI=1S/C35H45FN4O2/c1-2-31-34(38-33(37-31)19-25-8-4-3-5-9-25)26-14-16-39(17-15-26)21-28-22-40(32(35(41)42)18-24-10-6-11-24)23-30(28)27-12-7-13-29(36)20-27/h3-5,7-9,12-13,20,24,26,28,30,32H,2,6,10-11,14-19,21-23H2,1H3,(H,37,38)(H,41,42)/t28-,30+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50404274
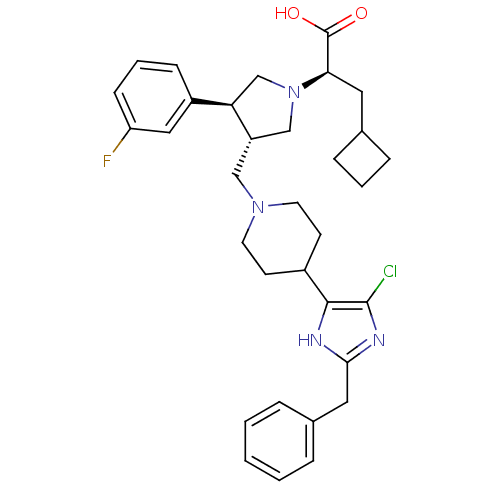
(CHEMBL2112557)Show SMILES OC(=O)[C@@H](CC1CCC1)N1C[C@H](CN2CCC(CC2)c2[nH]c(Cc3ccccc3)nc2Cl)[C@H](C1)c1cccc(F)c1 Show InChI InChI=1S/C33H40ClFN4O2/c34-32-31(36-30(37-32)17-23-6-2-1-3-7-23)24-12-14-38(15-13-24)19-26-20-39(29(33(40)41)16-22-8-4-9-22)21-28(26)25-10-5-11-27(35)18-25/h1-3,5-7,10-11,18,22,24,26,28-29H,4,8-9,12-17,19-21H2,(H,36,37)(H,40,41)/t26-,28+,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249302

((R)-2-((3S,4S)-3-((4-(2-benzyl-1H-imidazol-5-yl)pi...)Show SMILES OC(=O)[C@@H](C1CCCCC1)N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)[nH]2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C34H44N4O2/c39-34(40)33(28-14-8-3-9-15-28)38-23-29(30(24-38)26-12-6-2-7-13-26)22-37-18-16-27(17-19-37)31-21-35-32(36-31)20-25-10-4-1-5-11-25/h1-2,4-7,10-13,21,27-30,33H,3,8-9,14-20,22-24H2,(H,35,36)(H,39,40)/t29-,30+,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50404272
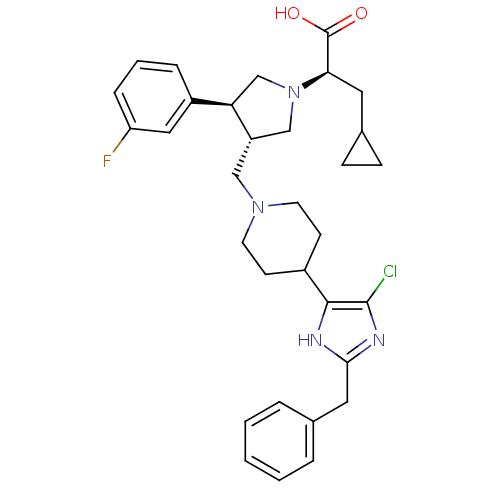
(CHEMBL2112552)Show SMILES OC(=O)[C@@H](CC1CC1)N1C[C@H](CN2CCC(CC2)c2[nH]c(Cc3ccccc3)nc2Cl)[C@H](C1)c1cccc(F)c1 Show InChI InChI=1S/C32H38ClFN4O2/c33-31-30(35-29(36-31)16-21-5-2-1-3-6-21)23-11-13-37(14-12-23)18-25-19-38(28(32(39)40)15-22-9-10-22)20-27(25)24-7-4-8-26(34)17-24/h1-8,17,22-23,25,27-28H,9-16,18-20H2,(H,35,36)(H,39,40)/t25-,27+,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50404278
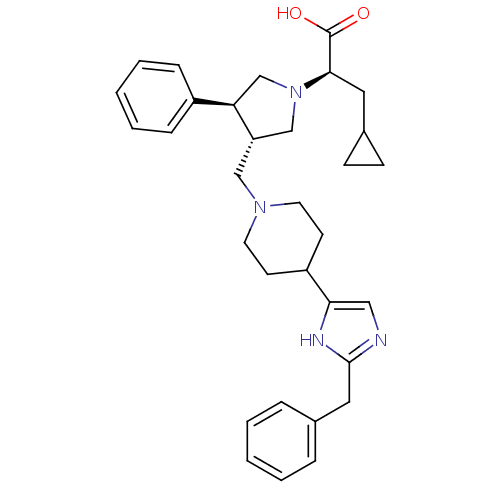
(CHEMBL2112560)Show SMILES OC(=O)[C@@H](CC1CC1)N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)[nH]2)[C@H](C1)c1ccccc1 Show InChI InChI=1S/C32H40N4O2/c37-32(38)30(17-24-11-12-24)36-21-27(28(22-36)25-9-5-2-6-10-25)20-35-15-13-26(14-16-35)29-19-33-31(34-29)18-23-7-3-1-4-8-23/h1-10,19,24,26-28,30H,11-18,20-22H2,(H,33,34)(H,37,38)/t27-,28+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50404277
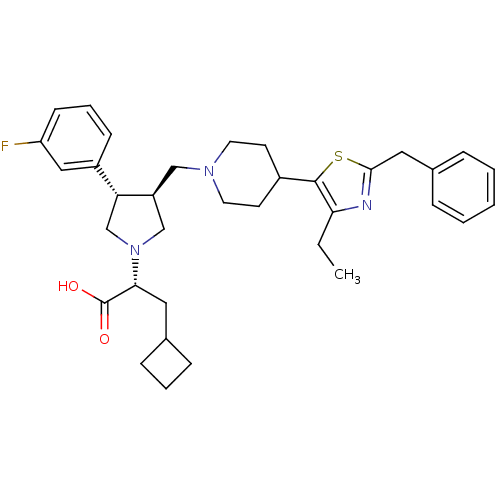
(CHEMBL2112553)Show SMILES CCc1nc(Cc2ccccc2)sc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](CC2CCC2)C(O)=O)CC1 Show InChI InChI=1S/C35H44FN3O2S/c1-2-31-34(42-33(37-31)19-25-8-4-3-5-9-25)26-14-16-38(17-15-26)21-28-22-39(32(35(40)41)18-24-10-6-11-24)23-30(28)27-12-7-13-29(36)20-27/h3-5,7-9,12-13,20,24,26,28,30,32H,2,6,10-11,14-19,21-23H2,1H3,(H,40,41)/t28-,30+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50404273
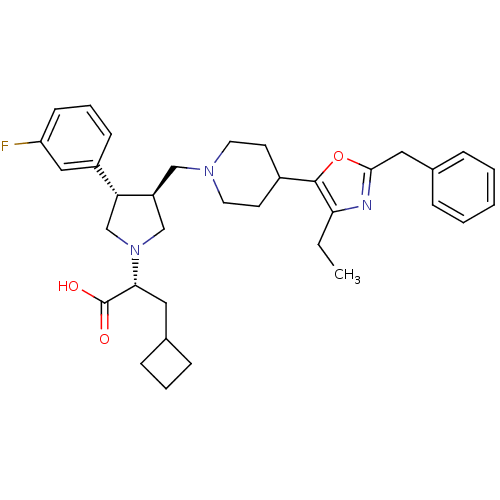
(CHEMBL2112556)Show SMILES CCc1nc(Cc2ccccc2)oc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](CC2CCC2)C(O)=O)CC1 Show InChI InChI=1S/C35H44FN3O3/c1-2-31-34(42-33(37-31)19-25-8-4-3-5-9-25)26-14-16-38(17-15-26)21-28-22-39(32(35(40)41)18-24-10-6-11-24)23-30(28)27-12-7-13-29(36)20-27/h3-5,7-9,12-13,20,24,26,28,30,32H,2,6,10-11,14-19,21-23H2,1H3,(H,40,41)/t28-,30+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50404280

(CHEMBL2112555)Show SMILES CCc1nc(Cc2ccccc2)sc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](C(C)C)C(O)=O)CC1 Show InChI InChI=1S/C33H42FN3O2S/c1-4-29-32(40-30(35-29)17-23-9-6-5-7-10-23)24-13-15-36(16-14-24)19-26-20-37(31(22(2)3)33(38)39)21-28(26)25-11-8-12-27(34)18-25/h5-12,18,22,24,26,28,31H,4,13-17,19-21H2,1-3H3,(H,38,39)/t26-,28+,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50404279

(CHEMBL2112554)Show SMILES OC(=O)[C@@H](C1CCCCC1)N1C[C@H](CN2CCC(CC2)c2csc(n2)-c2ccccc2)[C@H](C1)c1ccccc1 Show InChI InChI=1S/C33H41N3O2S/c37-33(38)31(26-12-6-2-7-13-26)36-21-28(29(22-36)24-10-4-1-5-11-24)20-35-18-16-25(17-19-35)30-23-39-32(34-30)27-14-8-3-9-15-27/h1,3-5,8-11,14-15,23,25-26,28-29,31H,2,6-7,12-13,16-22H2,(H,37,38)/t28-,29+,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249303

((R)-2-((3S,4S)-3-((4-(2-benzylthiazol-4-yl)piperid...)Show SMILES OC(=O)[C@@H](C1CCCCC1)N1C[C@H](CN2CCC(CC2)c2csc(Cc3ccccc3)n2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C34H43N3O2S/c38-34(39)33(28-14-8-3-9-15-28)37-22-29(30(23-37)26-12-6-2-7-13-26)21-36-18-16-27(17-19-36)31-24-40-32(35-31)20-25-10-4-1-5-11-25/h1-2,4-7,10-13,24,27-30,33H,3,8-9,14-23H2,(H,38,39)/t29-,30+,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249304
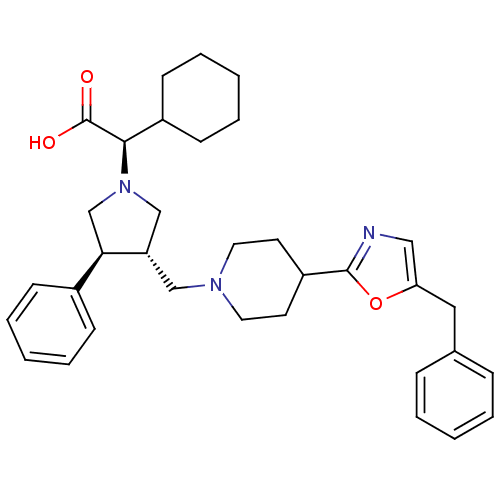
((R)-2-((3S,4S)-3-((4-(5-benzyloxazol-2-yl)piperidi...)Show SMILES OC(=O)[C@@H](C1CCCCC1)N1C[C@H](CN2CCC(CC2)c2ncc(Cc3ccccc3)o2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C34H43N3O3/c38-34(39)32(27-14-8-3-9-15-27)37-23-29(31(24-37)26-12-6-2-7-13-26)22-36-18-16-28(17-19-36)33-35-21-30(40-33)20-25-10-4-1-5-11-25/h1-2,4-7,10-13,21,27-29,31-32H,3,8-9,14-20,22-24H2,(H,38,39)/t29-,31+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249466

((R)-2-((3S,4S)-3-((4-(2-benzyloxazol-5-yl)piperidi...)Show SMILES OC(=O)[C@@H](CC1CC1)N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)o2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C32H39N3O3/c36-32(37)29(17-24-11-12-24)35-21-27(28(22-35)25-9-5-2-6-10-25)20-34-15-13-26(14-16-34)30-19-33-31(38-30)18-23-7-3-1-4-8-23/h1-10,19,24,26-29H,11-18,20-22H2,(H,36,37)/t27-,28+,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249430
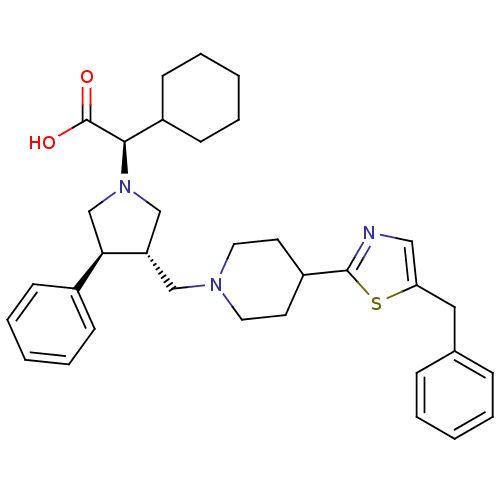
((R)-2-((3S,4S)-3-((4-(5-benzylthiazol-2-yl)piperid...)Show SMILES OC(=O)[C@@H](C1CCCCC1)N1C[C@H](CN2CCC(CC2)c2ncc(Cc3ccccc3)s2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C34H43N3O2S/c38-34(39)32(27-14-8-3-9-15-27)37-23-29(31(24-37)26-12-6-2-7-13-26)22-36-18-16-28(17-19-36)33-35-21-30(40-33)20-25-10-4-1-5-11-25/h1-2,4-7,10-13,21,27-29,31-32H,3,8-9,14-20,22-24H2,(H,38,39)/t29-,31+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249333
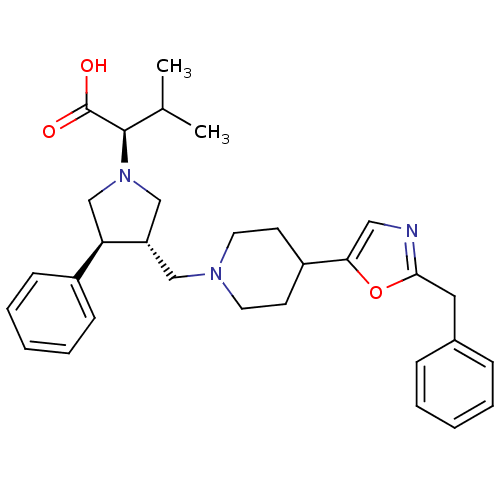
((R)-2-((3S,4S)-3-((4-(2-benzyloxazol-5-yl)piperidi...)Show SMILES CC(C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)o2)[C@H](C1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C31H39N3O3/c1-22(2)30(31(35)36)34-20-26(27(21-34)24-11-7-4-8-12-24)19-33-15-13-25(14-16-33)28-18-32-29(37-28)17-23-9-5-3-6-10-23/h3-12,18,22,25-27,30H,13-17,19-21H2,1-2H3,(H,35,36)/t26-,27+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249429
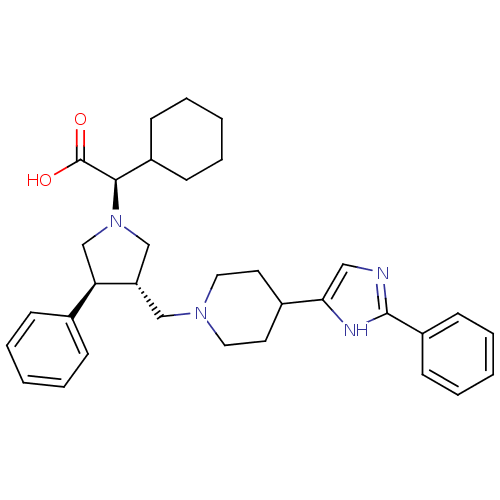
((R)-2-cyclohexyl-2-((3S,4S)-3-phenyl-4-((4-(2-phen...)Show SMILES OC(=O)[C@@H](C1CCCCC1)N1C[C@H](CN2CCC(CC2)c2cnc([nH]2)-c2ccccc2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C33H42N4O2/c38-33(39)31(26-12-6-2-7-13-26)37-22-28(29(23-37)24-10-4-1-5-11-24)21-36-18-16-25(17-19-36)30-20-34-32(35-30)27-14-8-3-9-15-27/h1,3-5,8-11,14-15,20,25-26,28-29,31H,2,6-7,12-13,16-19,21-23H2,(H,34,35)(H,38,39)/t28-,29+,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249332
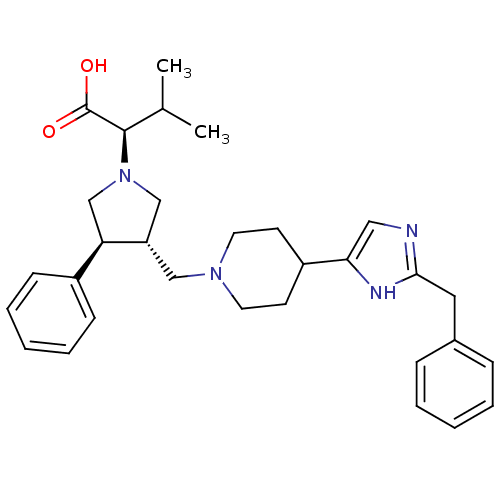
((R)-2-((3S,4S)-3-((4-(2-benzyl-1H-imidazol-5-yl)pi...)Show SMILES CC(C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cnc(Cc3ccccc3)[nH]2)[C@H](C1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C31H40N4O2/c1-22(2)30(31(36)37)35-20-26(27(21-35)24-11-7-4-8-12-24)19-34-15-13-25(14-16-34)28-18-32-29(33-28)17-23-9-5-3-6-10-23/h3-12,18,22,25-27,30H,13-17,19-21H2,1-2H3,(H,32,33)(H,36,37)/t26-,27+,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249395
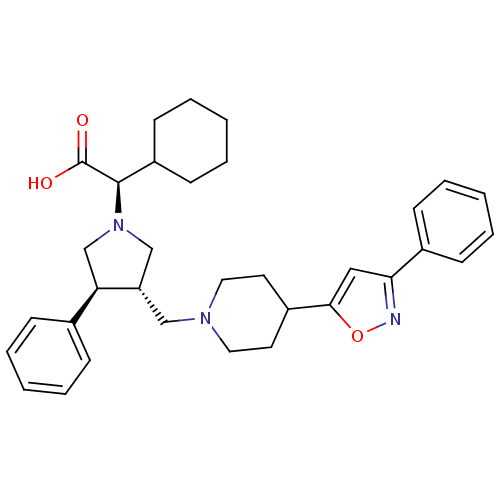
((R)-2-cyclohexyl-2-((3S,4S)-3-phenyl-4-((4-(3-phen...)Show SMILES OC(=O)[C@@H](C1CCCCC1)N1C[C@H](CN2CCC(CC2)c2cc(no2)-c2ccccc2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C33H41N3O3/c37-33(38)32(27-14-8-3-9-15-27)36-22-28(29(23-36)24-10-4-1-5-11-24)21-35-18-16-26(17-19-35)31-20-30(34-39-31)25-12-6-2-7-13-25/h1-2,4-7,10-13,20,26-29,32H,3,8-9,14-19,21-23H2,(H,37,38)/t28-,29+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against C-C chemokine receptor type 5 using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066778
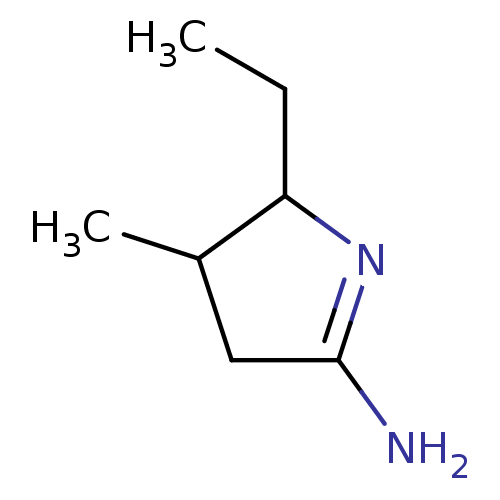
(5-Ethyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hyd...)Show InChI InChI=1S/C7H14N2/c1-3-6-5(2)4-7(8)9-6/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249428

((R)-2-cyclohexyl-2-((3S,4S)-3-phenyl-4-((4-(4-phen...)Show SMILES OC(=O)[C@@H](C1CCCCC1)N1C[C@H](CN2CCC(CC2)c2nc(c[nH]2)-c2ccccc2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C33H42N4O2/c38-33(39)31(26-14-8-3-9-15-26)37-22-28(29(23-37)24-10-4-1-5-11-24)21-36-18-16-27(17-19-36)32-34-20-30(35-32)25-12-6-2-7-13-25/h1-2,4-7,10-13,20,26-29,31H,3,8-9,14-19,21-23H2,(H,34,35)(H,38,39)/t28-,29+,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50249396

((R)-2-cyclohexyl-2-((3S,4S)-3-phenyl-4-((4-(4-phen...)Show SMILES OC(=O)[C@@H](C1CCCCC1)N1C[C@H](CN2CCC(CC2)c2nocc2-c2ccccc2)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C33H41N3O3/c37-33(38)32(27-14-8-3-9-15-27)36-21-28(29(22-36)24-10-4-1-5-11-24)20-35-18-16-26(17-19-35)31-30(23-39-34-31)25-12-6-2-7-13-25/h1-2,4-7,10-13,23,26-29,32H,3,8-9,14-22H2,(H,37,38)/t28-,29+,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human C-C chemokine receptor type 5 assayed using [125I]-MIP-1 alpha as radioligand |
Bioorg Med Chem Lett 14: 3419-24 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.078
BindingDB Entry DOI: 10.7270/Q2X63MD1 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50066778

(5-Ethyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hyd...)Show InChI InChI=1S/C7H14N2/c1-3-6-5(2)4-7(8)9-6/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150924
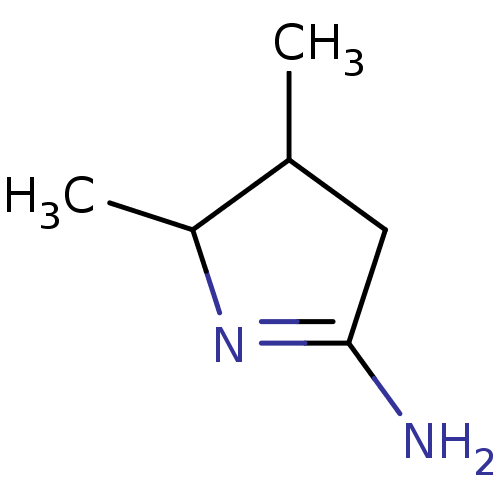
(4,5-Dimethyl-pyrrolidin-(2Z)-ylideneamine | CHEMBL...)Show InChI InChI=1S/C6H12N2/c1-4-3-6(7)8-5(4)2/h4-5H,3H2,1-2H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150933
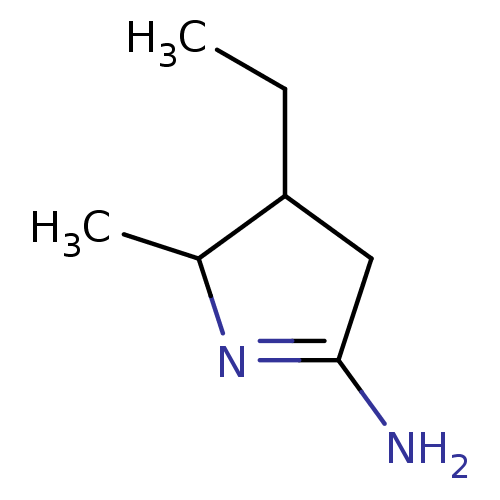
(4-Ethyl-5-methyl-pyrrolidin-(2Z)-ylideneamine | CH...)Show InChI InChI=1S/C7H14N2/c1-3-6-4-7(8)9-5(6)2/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50155790
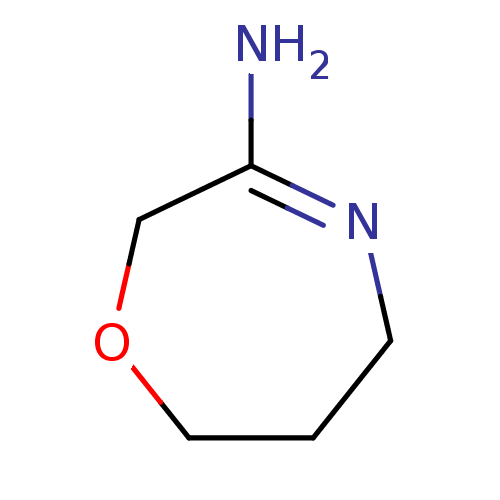
(CHEMBL362772 | [1,4]Oxazepan-(3E)-ylideneamine)Show InChI InChI=1S/C5H10N2O/c6-5-4-8-3-1-2-7-5/h1-4H2,(H2,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50155790

(CHEMBL362772 | [1,4]Oxazepan-(3E)-ylideneamine)Show InChI InChI=1S/C5H10N2O/c6-5-4-8-3-1-2-7-5/h1-4H2,(H2,6,7) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50150933

(4-Ethyl-5-methyl-pyrrolidin-(2Z)-ylideneamine | CH...)Show InChI InChI=1S/C7H14N2/c1-3-6-4-7(8)9-5(6)2/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50155789
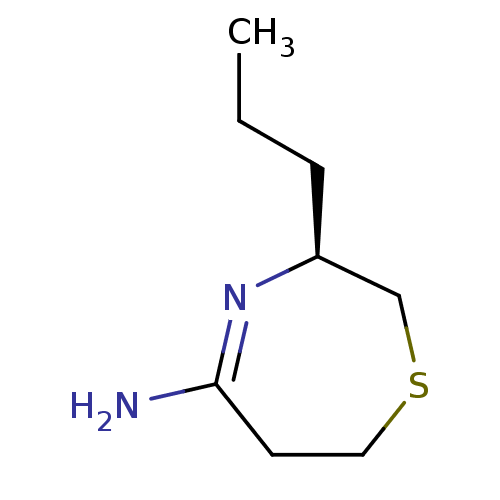
((S)-3-Propyl-[1,4]thiazepan-(5E)-ylideneamine | CH...)Show InChI InChI=1S/C8H16N2S/c1-2-3-7-6-11-5-4-8(9)10-7/h7H,2-6H2,1H3,(H2,9,10)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066780

(4-Methyl-pyrrolidin-(2E)-ylideneamine; hydrochlori...)Show InChI InChI=1S/C5H10N2/c1-4-2-5(6)7-3-4/h4H,2-3H2,1H3,(H2,6,7) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50155788
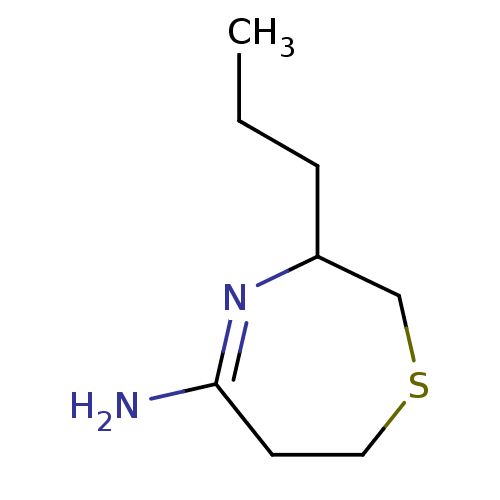
(3-Propyl-[1,4]thiazepan-(5E)-ylideneamine | CHEMBL...)Show InChI InChI=1S/C8H16N2S/c1-2-3-7-6-11-5-4-8(9)10-7/h7H,2-6H2,1H3,(H2,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50150924

(4,5-Dimethyl-pyrrolidin-(2Z)-ylideneamine | CHEMBL...)Show InChI InChI=1S/C6H12N2/c1-4-3-6(7)8-5(4)2/h4-5H,3H2,1-2H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50155786

(3-Ethyl-[1,4]thiazepan-(5E)-ylideneamine | CHEMBL1...)Show InChI InChI=1S/C7H14N2S/c1-2-6-5-10-4-3-7(8)9-6/h6H,2-5H2,1H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150935
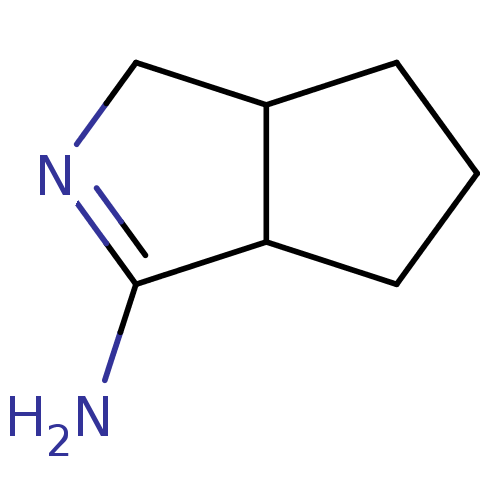
(CHEMBL365849 | Hexahydro-cyclopenta[c]pyrrol-(1Z)-...)Show InChI InChI=1S/C7H12N2/c8-7-6-3-1-2-5(6)4-9-7/h5-6H,1-4H2,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50155782

(3-Isobutyl-[1,4]thiazepan-(5E)-ylideneamine | CHEM...)Show InChI InChI=1S/C9H18N2S/c1-7(2)5-8-6-12-4-3-9(10)11-8/h7-8H,3-6H2,1-2H3,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50155789

((S)-3-Propyl-[1,4]thiazepan-(5E)-ylideneamine | CH...)Show InChI InChI=1S/C8H16N2S/c1-2-3-7-6-11-5-4-8(9)10-7/h7H,2-6H2,1H3,(H2,9,10)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50155790

(CHEMBL362772 | [1,4]Oxazepan-(3E)-ylideneamine)Show InChI InChI=1S/C5H10N2O/c6-5-4-8-3-1-2-7-5/h1-4H2,(H2,6,7) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Endothelial nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50155787

(3-Butyl-[1,4]thiazepan-(5E)-ylideneamine | CHEMBL1...)Show InChI InChI=1S/C9H18N2S/c1-2-3-4-8-7-12-6-5-9(10)11-8/h8H,2-7H2,1H3,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066783
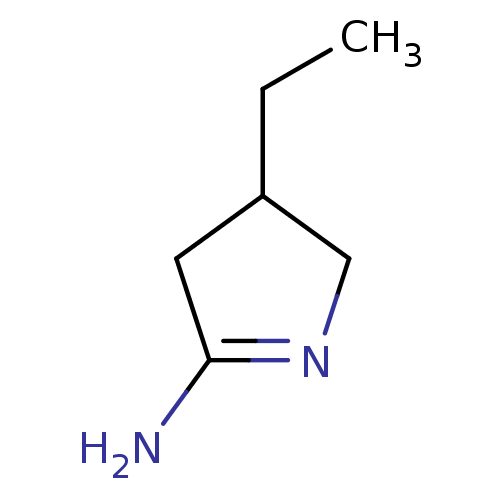
(4-Ethyl-pyrrolidin-(2E)-ylideneamine; hydrochlorid...)Show InChI InChI=1S/C6H12N2/c1-2-5-3-6(7)8-4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50066778

(5-Ethyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hyd...)Show InChI InChI=1S/C7H14N2/c1-3-6-5(2)4-7(8)9-6/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Endothelial nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50155786

(3-Ethyl-[1,4]thiazepan-(5E)-ylideneamine | CHEMBL1...)Show InChI InChI=1S/C7H14N2S/c1-2-6-5-10-4-3-7(8)9-6/h6H,2-5H2,1H3,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 5907-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.019
BindingDB Entry DOI: 10.7270/Q2JQ10HX |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50150924

(4,5-Dimethyl-pyrrolidin-(2Z)-ylideneamine | CHEMBL...)Show InChI InChI=1S/C6H12N2/c1-4-3-6(7)8-5(4)2/h4-5H,3H2,1-2H3,(H2,7,8) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Endothelial nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150930

(4-Ethyl-3-methyl-pyrrolidin-(2Z)-ylideneamine | CH...)Show InChI InChI=1S/C7H14N2/c1-3-6-4-9-7(8)5(6)2/h5-6H,3-4H2,1-2H3,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150928

(3,4-Dimethyl-pyrrolidin-(2Z)-ylideneamine | CHEMBL...)Show InChI InChI=1S/C6H12N2/c1-4-3-8-6(7)5(4)2/h4-5H,3H2,1-2H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50150929

(CHEMBL185441 | Octahydro-isoindol-(1Z)-ylideneamin...)Show InChI InChI=1S/C8H14N2/c9-8-7-4-2-1-3-6(7)5-10-8/h6-7H,1-5H2,(H2,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity against Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 14: 4539-44 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.033
BindingDB Entry DOI: 10.7270/Q218377V |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50290814

(5-Chloro-3-morpholin-4-ylmethyl-3H-benzooxazol-2-o...)Show InChI InChI=1S/C12H13ClN2O3/c13-9-1-2-11-10(7-9)15(12(16)18-11)8-14-3-5-17-6-4-14/h1-2,7H,3-6,8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Inducible nitric oxide synthase |
Bioorg Med Chem Lett 7: 2887-2892 (1997)
Article DOI: 10.1016/S0960-894X(97)10101-9
BindingDB Entry DOI: 10.7270/Q28G8KQD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data