 Found 141 hits with Last Name = 'abouzid' and Initial = 'kam'
Found 141 hits with Last Name = 'abouzid' and Initial = 'kam' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Putative penicillin-binding protein 2B
(Staphylococcus aureus) | BDBM50611988
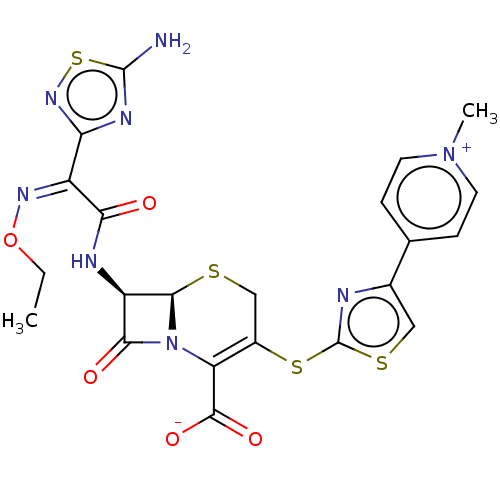
(CEFTAROLINE | Ceftaroline | T 91825 | T-91825)Show SMILES [H][C@]12SCC(Sc3nc(cs3)-c3cc[n+](C)cc3)=C(N1C(=O)[C@H]2NC(=O)C(=N/OCC)\c1nsc(N)n1)C([O-])=O |r,c:19| | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peptidase
(Staphylococcus aureus) | BDBM50611988

(CEFTAROLINE | Ceftaroline | T 91825 | T-91825)Show SMILES [H][C@]12SCC(Sc3nc(cs3)-c3cc[n+](C)cc3)=C(N1C(=O)[C@H]2NC(=O)C(=N/OCC)\c1nsc(N)n1)C([O-])=O |r,c:19| | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase ROS
(Homo sapiens (Human)) | BDBM158154

(US10081622, Compound 11 | US10370379, Entrectinib ...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2n[nH]c3ccc(Cc4cc(F)cc(F)c4)cc23)c(NC2CCOCC2)c1 Show InChI InChI=1S/C31H34F2N6O2/c1-38-8-10-39(11-9-38)25-3-4-26(29(19-25)34-24-6-12-41-13-7-24)31(40)35-30-27-17-20(2-5-28(27)36-37-30)14-21-15-22(32)18-23(33)16-21/h2-5,15-19,24,34H,6-14H2,1H3,(H2,35,36,37,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.254 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.259 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50596396

(CHEMBL5201174)Show SMILES CCc1nc(C(N)=O)c(Cc2ccc(N3CCC(CC3)N3CCN(C)CC3)c(OC)c2)nc1CN1CCOCC1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR catalytic domain expressed in insect cells |
Eur J Med Chem 142: 131-151 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.023
BindingDB Entry DOI: 10.7270/Q2JH3PWT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR L858R mutant expressed in mouse Ba/F3 cells |
Eur J Med Chem 142: 131-151 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.023
BindingDB Entry DOI: 10.7270/Q2JH3PWT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50406868

(BIBW2992 | CHEMBL2347958)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR L858R mutant expressed in mouse Ba/F3 cells |
Eur J Med Chem 142: 131-151 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.023
BindingDB Entry DOI: 10.7270/Q2JH3PWT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50406868

(BIBW2992 | CHEMBL2347958)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR catalytic domain expressed in insect cells |
Eur J Med Chem 142: 131-151 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.023
BindingDB Entry DOI: 10.7270/Q2JH3PWT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50204093
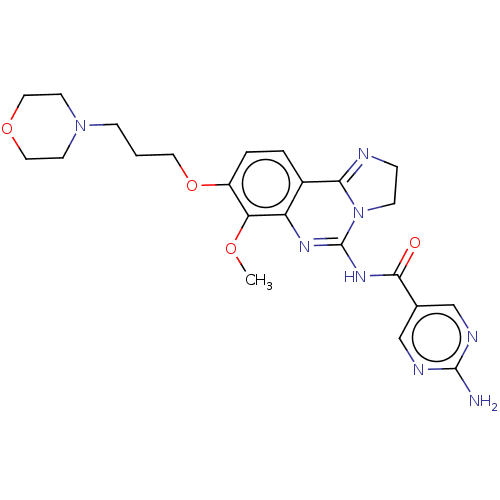
(BAY 80-6946 | BAY-80-6946 | Copanlisib)Show SMILES COc1c(OCCCN2CCOCC2)ccc2C3=NCCN3C(NC(=O)c3cnc(N)nc3)=Nc12 |c:35,t:18| Show InChI InChI=1S/C23H28N8O4/c1-33-19-17(35-10-2-6-30-8-11-34-12-9-30)4-3-16-18(19)28-23(31-7-5-25-20(16)31)29-21(32)15-13-26-22(24)27-14-15/h3-4,13-14H,2,5-12H2,1H3,(H2,24,26,27)(H,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111718
BindingDB Entry DOI: 10.7270/Q23J3HCB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50468245
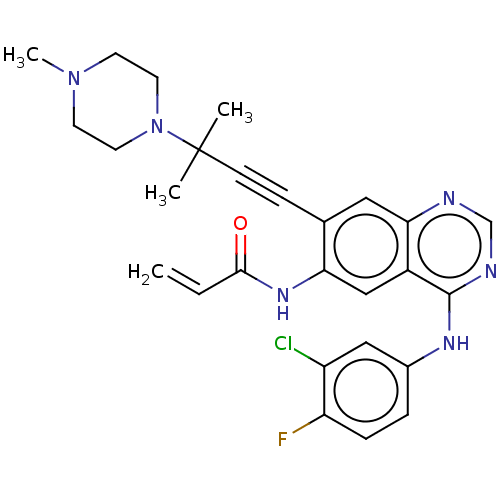
(AV-412 | CHEMBL2138625 | MP-412)Show SMILES CN1CCN(CC1)C(C)(C)C#Cc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)C=C Show InChI InChI=1S/C27H28ClFN6O/c1-5-25(36)33-23-16-20-24(30-17-31-26(20)32-19-6-7-22(29)21(28)15-19)14-18(23)8-9-27(2,3)35-12-10-34(4)11-13-35/h5-7,14-17H,1,10-13H2,2-4H3,(H,33,36)(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR L858R mutant expressed in mouse Ba/F3 cells |
Eur J Med Chem 142: 131-151 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.023
BindingDB Entry DOI: 10.7270/Q2JH3PWT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50204093

(BAY 80-6946 | BAY-80-6946 | Copanlisib)Show SMILES COc1c(OCCCN2CCOCC2)ccc2C3=NCCN3C(NC(=O)c3cnc(N)nc3)=Nc12 |c:35,t:18| Show InChI InChI=1S/C23H28N8O4/c1-33-19-17(35-10-2-6-30-8-11-34-12-9-30)4-3-16-18(19)28-23(31-7-5-25-20(16)31)29-21(32)15-13-26-22(24)27-14-15/h3-4,13-14H,2,5-12H2,1H3,(H2,24,26,27)(H,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111718
BindingDB Entry DOI: 10.7270/Q23J3HCB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM185674
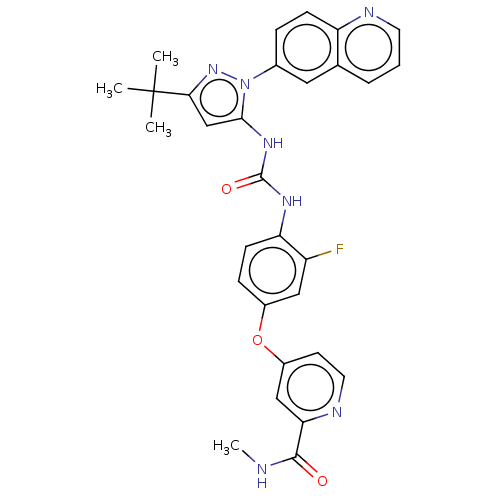
(4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3cc(nn3-c3ccc4ncccc4c3)C(C)(C)C)c(F)c2)ccn1 Show InChI InChI=1S/C30H28FN7O3/c1-30(2,3)26-17-27(38(37-26)19-7-9-23-18(14-19)6-5-12-33-23)36-29(40)35-24-10-8-20(15-22(24)31)41-21-11-13-34-25(16-21)28(39)32-4/h5-17H,1-4H3,(H,32,39)(H2,35,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50468245

(AV-412 | CHEMBL2138625 | MP-412)Show SMILES CN1CCN(CC1)C(C)(C)C#Cc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)C=C Show InChI InChI=1S/C27H28ClFN6O/c1-5-25(36)33-23-16-20-24(30-17-31-26(20)32-19-6-7-22(29)21(28)15-19)14-18(23)8-9-27(2,3)35-12-10-34(4)11-13-35/h5-7,14-17H,1,10-13H2,2-4H3,(H,33,36)(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR T790M mutant expressed in mouse Ba/F3 cells |
Eur J Med Chem 142: 131-151 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.023
BindingDB Entry DOI: 10.7270/Q2JH3PWT |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM158154

(US10081622, Compound 11 | US10370379, Entrectinib ...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2n[nH]c3ccc(Cc4cc(F)cc(F)c4)cc23)c(NC2CCOCC2)c1 Show InChI InChI=1S/C31H34F2N6O2/c1-38-8-10-39(11-9-38)25-3-4-26(29(19-25)34-24-6-12-41-13-7-24)31(40)35-30-27-17-20(2-5-28(27)36-37-30)14-21-15-22(32)18-23(33)16-21/h2-5,15-19,24,34H,6-14H2,1H3,(H2,35,36,37,40) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM158154

(US10081622, Compound 11 | US10370379, Entrectinib ...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2n[nH]c3ccc(Cc4cc(F)cc(F)c4)cc23)c(NC2CCOCC2)c1 Show InChI InChI=1S/C31H34F2N6O2/c1-38-8-10-39(11-9-38)25-3-4-26(29(19-25)34-24-6-12-41-13-7-24)31(40)35-30-27-17-20(2-5-28(27)36-37-30)14-21-15-22(32)18-23(33)16-21/h2-5,15-19,24,34H,6-14H2,1H3,(H2,35,36,37,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM158154

(US10081622, Compound 11 | US10370379, Entrectinib ...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2n[nH]c3ccc(Cc4cc(F)cc(F)c4)cc23)c(NC2CCOCC2)c1 Show InChI InChI=1S/C31H34F2N6O2/c1-38-8-10-39(11-9-38)25-3-4-26(29(19-25)34-24-6-12-41-13-7-24)31(40)35-30-27-17-20(2-5-28(27)36-37-30)14-21-15-22(32)18-23(33)16-21/h2-5,15-19,24,34H,6-14H2,1H3,(H2,35,36,37,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Peptidase
(Staphylococcus aureus) | BDBM50611989
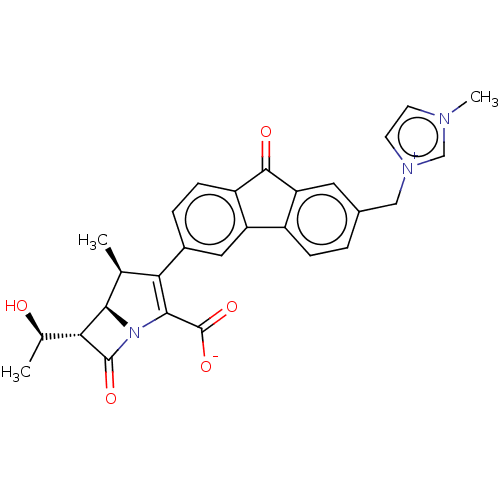
(CHEMBL5271107)Show SMILES [H][C@]1([C@@H](C)O)C(=O)N2C(C([O-])=O)=C([C@H](C)[C@]12[H])c1ccc2C(=O)c3cc(C[n+]4ccn(C)c4)ccc3-c2c1 |r,c:11| | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185149
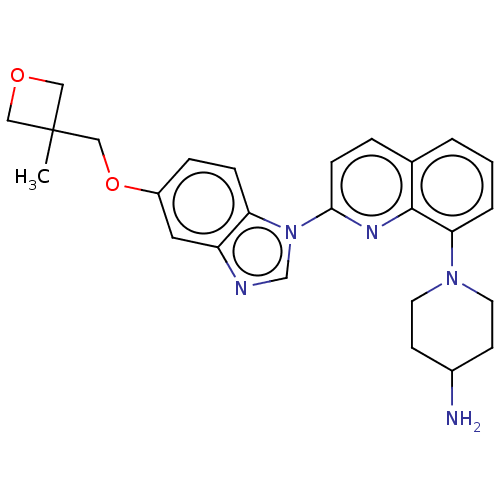
(1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...)Show SMILES CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 Show InChI InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50468245

(AV-412 | CHEMBL2138625 | MP-412)Show SMILES CN1CCN(CC1)C(C)(C)C#Cc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)C=C Show InChI InChI=1S/C27H28ClFN6O/c1-5-25(36)33-23-16-20-24(30-17-31-26(20)32-19-6-7-22(29)21(28)15-19)14-18(23)8-9-27(2,3)35-12-10-34(4)11-13-35/h5-7,14-17H,1,10-13H2,2-4H3,(H,33,36)(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR catalytic domain expressed in insect cells |
Eur J Med Chem 142: 131-151 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.023
BindingDB Entry DOI: 10.7270/Q2JH3PWT |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50596396

(CHEMBL5201174)Show SMILES CCc1nc(C(N)=O)c(Cc2ccc(N3CCC(CC3)N3CCN(C)CC3)c(OC)c2)nc1CN1CCOCC1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM158154

(US10081622, Compound 11 | US10370379, Entrectinib ...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2n[nH]c3ccc(Cc4cc(F)cc(F)c4)cc23)c(NC2CCOCC2)c1 Show InChI InChI=1S/C31H34F2N6O2/c1-38-8-10-39(11-9-38)25-3-4-26(29(19-25)34-24-6-12-41-13-7-24)31(40)35-30-27-17-20(2-5-28(27)36-37-30)14-21-15-22(32)18-23(33)16-21/h2-5,15-19,24,34H,6-14H2,1H3,(H2,35,36,37,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50596396

(CHEMBL5201174)Show SMILES CCc1nc(C(N)=O)c(Cc2ccc(N3CCC(CC3)N3CCN(C)CC3)c(OC)c2)nc1CN1CCOCC1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185674

(4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3cc(nn3-c3ccc4ncccc4c3)C(C)(C)C)c(F)c2)ccn1 Show InChI InChI=1S/C30H28FN7O3/c1-30(2,3)26-17-27(38(37-26)19-7-9-23-18(14-19)6-5-12-33-23)36-29(40)35-24-10-8-20(15-22(24)31)41-21-11-13-34-25(16-21)28(39)32-4/h5-17H,1-4H3,(H,32,39)(H2,35,36,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185149

(1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...)Show SMILES CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 Show InChI InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50468247
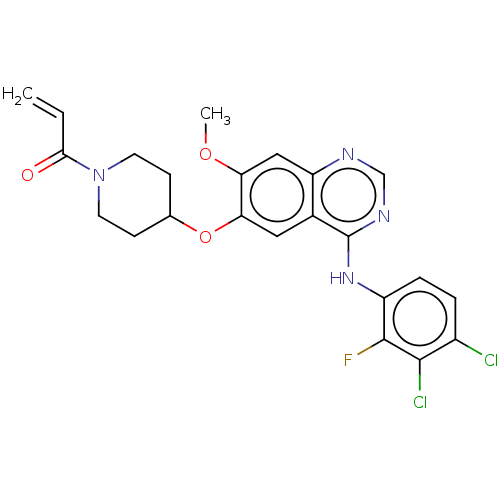
(HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)c(Cl)c3F)c2cc1OC1CCN(CC1)C(=O)C=C Show InChI InChI=1S/C23H21Cl2FN4O3/c1-3-20(31)30-8-6-13(7-9-30)33-19-10-14-17(11-18(19)32-2)27-12-28-23(14)29-16-5-4-15(24)21(25)22(16)26/h3-5,10-13H,1,6-9H2,2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR T790M/L858R mutant expressed in mouse Ba/F3 cells |
Eur J Med Chem 142: 131-151 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.023
BindingDB Entry DOI: 10.7270/Q2JH3PWT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50468245

(AV-412 | CHEMBL2138625 | MP-412)Show SMILES CN1CCN(CC1)C(C)(C)C#Cc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)C=C Show InChI InChI=1S/C27H28ClFN6O/c1-5-25(36)33-23-16-20-24(30-17-31-26(20)32-19-6-7-22(29)21(28)15-19)14-18(23)8-9-27(2,3)35-12-10-34(4)11-13-35/h5-7,14-17H,1,10-13H2,2-4H3,(H,33,36)(H,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR T790M/L858R mutant expressed in mouse Ba/F3 cells |
Eur J Med Chem 142: 131-151 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.023
BindingDB Entry DOI: 10.7270/Q2JH3PWT |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50308060
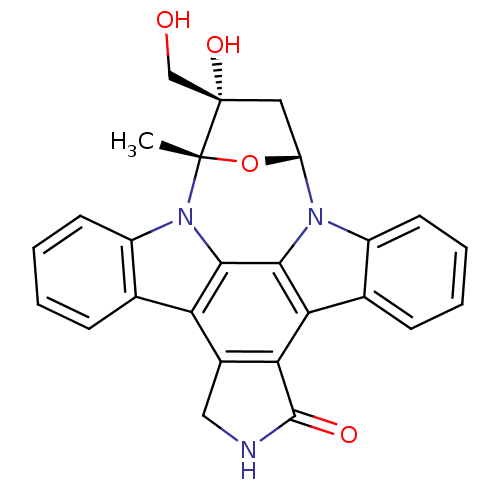
(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111718
BindingDB Entry DOI: 10.7270/Q23J3HCB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111718
BindingDB Entry DOI: 10.7270/Q23J3HCB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50468247

(HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)c(Cl)c3F)c2cc1OC1CCN(CC1)C(=O)C=C Show InChI InChI=1S/C23H21Cl2FN4O3/c1-3-20(31)30-8-6-13(7-9-30)33-19-10-14-17(11-18(19)32-2)27-12-28-23(14)29-16-5-4-15(24)21(25)22(16)26/h3-5,10-13H,1,6-9H2,2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR catalytic domain expressed in insect cells |
Eur J Med Chem 142: 131-151 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.023
BindingDB Entry DOI: 10.7270/Q2JH3PWT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50204093

(BAY 80-6946 | BAY-80-6946 | Copanlisib)Show SMILES COc1c(OCCCN2CCOCC2)ccc2C3=NCCN3C(NC(=O)c3cnc(N)nc3)=Nc12 |c:35,t:18| Show InChI InChI=1S/C23H28N8O4/c1-33-19-17(35-10-2-6-30-8-11-34-12-9-30)4-3-16-18(19)28-23(31-7-5-25-20(16)31)29-21(32)15-13-26-22(24)27-14-15/h3-4,13-14H,2,5-12H2,1H3,(H2,24,26,27)(H,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111718
BindingDB Entry DOI: 10.7270/Q23J3HCB |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50468247

(HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)c(Cl)c3F)c2cc1OC1CCN(CC1)C(=O)C=C Show InChI InChI=1S/C23H21Cl2FN4O3/c1-3-20(31)30-8-6-13(7-9-30)33-19-10-14-17(11-18(19)32-2)27-12-28-23(14)29-16-5-4-15(24)21(25)22(16)26/h3-5,10-13H,1,6-9H2,2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR T790M mutant expressed in mouse Ba/F3 cells |
Eur J Med Chem 142: 131-151 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.023
BindingDB Entry DOI: 10.7270/Q2JH3PWT |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50515042
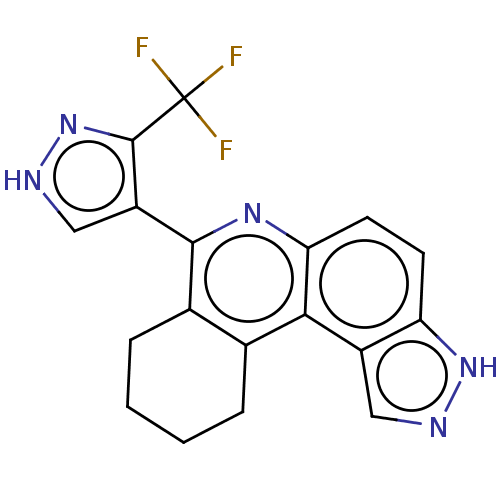
(CHEMBL4593398)Show SMILES FC(F)(F)c1n[nH]cc1-c1nc2ccc3[nH]ncc3c2c2CCCCc12 Show InChI InChI=1S/C18H14F3N5/c19-18(20,21)17-12(8-23-26-17)16-10-4-2-1-3-9(10)15-11-7-22-25-13(11)5-6-14(15)24-16/h5-8H,1-4H2,(H,22,25)(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM136597
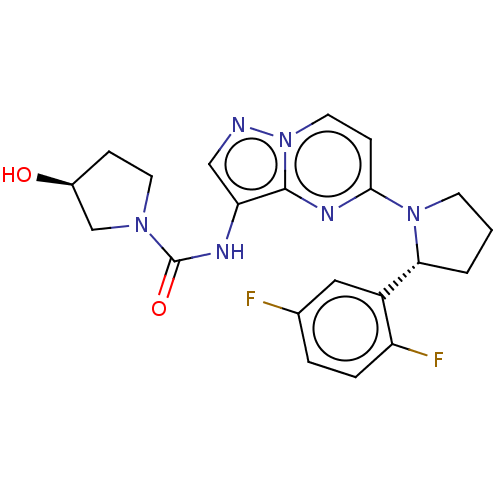
(US10005783, 14 | US10047097, 14 | US10774085, Exam...)Show SMILES O[C@H]1CCN(C1)C(=O)Nc1cnn2ccc(nc12)N1CCC[C@@H]1c1cc(F)ccc1F |r| Show InChI InChI=1S/C21H22F2N6O2/c22-13-3-4-16(23)15(10-13)18-2-1-7-28(18)19-6-9-29-20(26-19)17(11-24-29)25-21(31)27-8-5-14(30)12-27/h3-4,6,9-11,14,18,30H,1-2,5,7-8,12H2,(H,25,31)/t14-,18+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM136597

(US10005783, 14 | US10047097, 14 | US10774085, Exam...)Show SMILES O[C@H]1CCN(C1)C(=O)Nc1cnn2ccc(nc12)N1CCC[C@@H]1c1cc(F)ccc1F |r| Show InChI InChI=1S/C21H22F2N6O2/c22-13-3-4-16(23)15(10-13)18-2-1-7-28(18)19-6-9-29-20(26-19)17(11-24-29)25-21(31)27-8-5-14(30)12-27/h3-4,6,9-11,14,18,30H,1-2,5,7-8,12H2,(H,25,31)/t14-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50315213
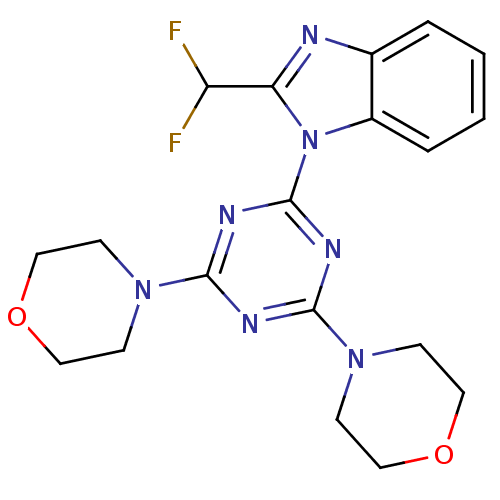
(2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...)Show SMILES FC(F)c1nc2ccccc2n1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O2/c20-15(21)16-22-13-3-1-2-4-14(13)28(16)19-24-17(26-5-9-29-10-6-26)23-18(25-19)27-7-11-30-12-8-27/h1-4,15H,5-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Competitive inhibition of PI3Kdelta (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111718
BindingDB Entry DOI: 10.7270/Q23J3HCB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM136597

(US10005783, 14 | US10047097, 14 | US10774085, Exam...)Show SMILES O[C@H]1CCN(C1)C(=O)Nc1cnn2ccc(nc12)N1CCC[C@@H]1c1cc(F)ccc1F |r| Show InChI InChI=1S/C21H22F2N6O2/c22-13-3-4-16(23)15(10-13)18-2-1-7-28(18)19-6-9-29-20(26-19)17(11-24-29)25-21(31)27-8-5-14(30)12-27/h3-4,6,9-11,14,18,30H,1-2,5,7-8,12H2,(H,25,31)/t14-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50468247

(HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)c(Cl)c3F)c2cc1OC1CCN(CC1)C(=O)C=C Show InChI InChI=1S/C23H21Cl2FN4O3/c1-3-20(31)30-8-6-13(7-9-30)33-19-10-14-17(11-18(19)32-2)27-12-28-23(14)29-16-5-4-15(24)21(25)22(16)26/h3-5,10-13H,1,6-9H2,2H3,(H,27,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human HER2 catalytic domain expressed in insect cells |
Eur J Med Chem 142: 131-151 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.023
BindingDB Entry DOI: 10.7270/Q2JH3PWT |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM185674

(4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3cc(nn3-c3ccc4ncccc4c3)C(C)(C)C)c(F)c2)ccn1 Show InChI InChI=1S/C30H28FN7O3/c1-30(2,3)26-17-27(38(37-26)19-7-9-23-18(14-19)6-5-12-33-23)36-29(40)35-24-10-8-20(15-22(24)31)41-21-11-13-34-25(16-21)28(39)32-4/h5-17H,1-4H3,(H,32,39)(H2,35,36,40) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM112499
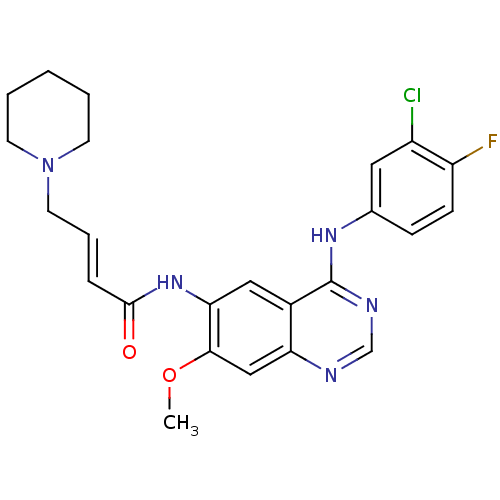
(DACOMITINIB | US8623883, No. 2 | WO2022090481, Exa...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CN1CCCCC1 Show InChI InChI=1S/C24H25ClFN5O2/c1-33-22-14-20-17(24(28-15-27-20)29-16-7-8-19(26)18(25)12-16)13-21(22)30-23(32)6-5-11-31-9-3-2-4-10-31/h5-8,12-15H,2-4,9-11H2,1H3,(H,30,32)(H,27,28,29)/b6-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR catalytic domain expressed in insect cells |
Eur J Med Chem 142: 131-151 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.023
BindingDB Entry DOI: 10.7270/Q2JH3PWT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50204093

(BAY 80-6946 | BAY-80-6946 | Copanlisib)Show SMILES COc1c(OCCCN2CCOCC2)ccc2C3=NCCN3C(NC(=O)c3cnc(N)nc3)=Nc12 |c:35,t:18| Show InChI InChI=1S/C23H28N8O4/c1-33-19-17(35-10-2-6-30-8-11-34-12-9-30)4-3-16-18(19)28-23(31-7-5-25-20(16)31)29-21(32)15-13-26-22(24)27-14-15/h3-4,13-14H,2,5-12H2,1H3,(H2,24,26,27)(H,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111718
BindingDB Entry DOI: 10.7270/Q23J3HCB |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185149

(1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...)Show SMILES CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 Show InChI InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50519385
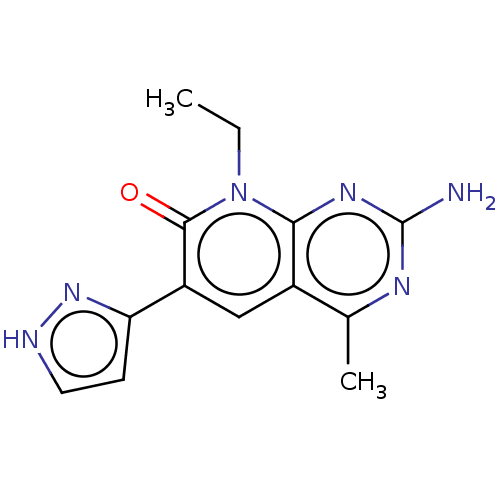
(SAR-245409 | SAR245409 | Voxtalisib | XL-765 | XL7...)Show InChI InChI=1S/C13H14N6O/c1-3-19-11-8(7(2)16-13(14)17-11)6-9(12(19)20)10-4-5-15-18-10/h4-6H,3H2,1-2H3,(H,15,18)(H2,14,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110gamma (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111718
BindingDB Entry DOI: 10.7270/Q23J3HCB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data