

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50355500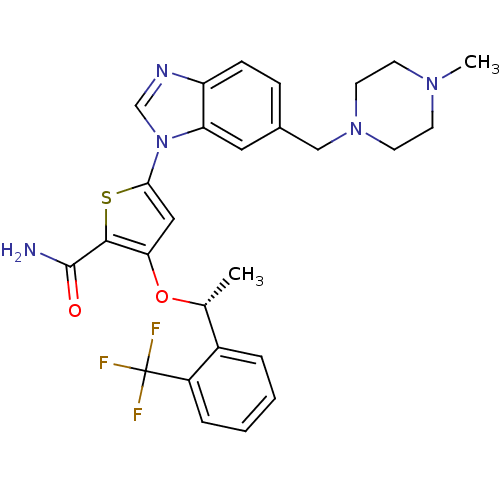 (CHEMBL1908394 | US9695172, GSK461364) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00533 BindingDB Entry DOI: 10.7270/Q23X8BM3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069292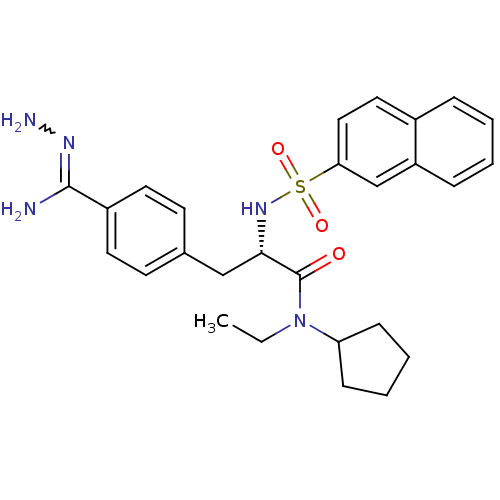 (CHEMBL156082 | N-ethyl-N-cyclopentyl-3-(4-hydrazon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069293 (CHEMBL440188 | N-methyl-N-n-butyl-3-(4-hydrazonofo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069296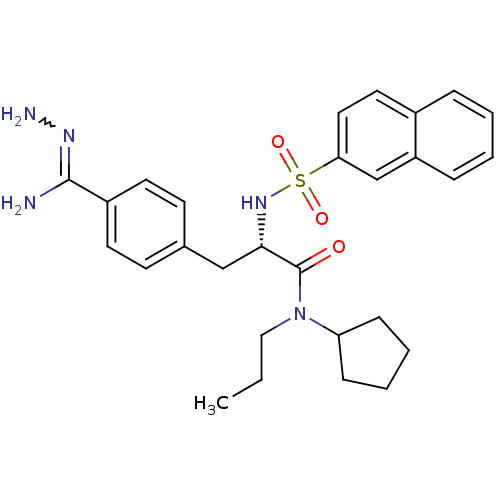 (CHEMBL347371 | N-(n-propyl)-N-cyclopentyl-3-(4-hyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069298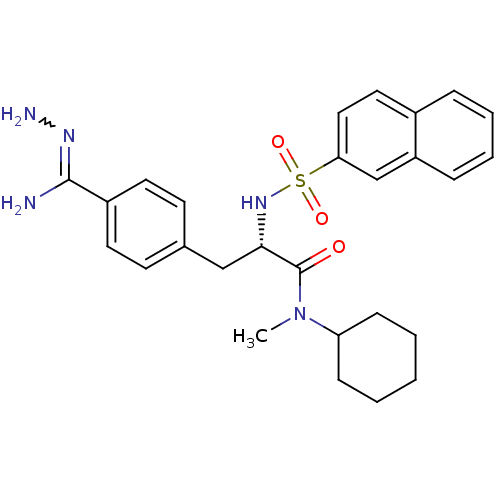 (CHEMBL434678 | N-methyl-N-cyclohexyl-3-(4-hydrazon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069299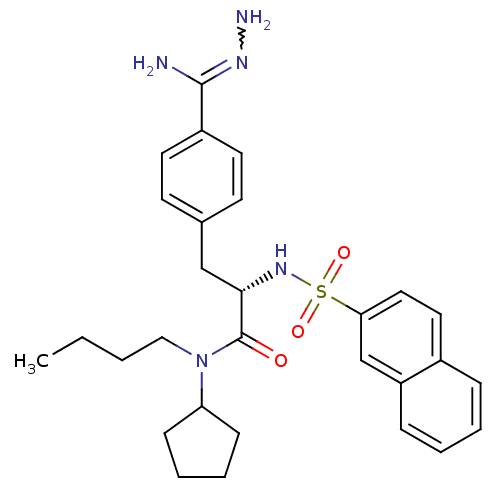 (CHEMBL155317 | N-(n-butyl)-N-cyclopentyl-3-(4-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069295 (CHEMBL348175 | N-methyl-N-cyclopropyl-3-(4-hydrazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069300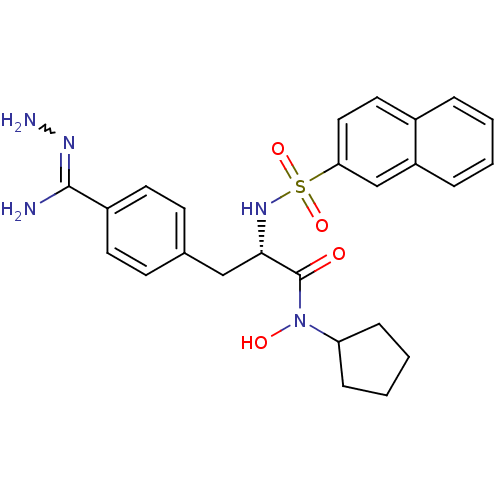 (CHEMBL350901 | N-hydroxy-N-cyclopentyl-3-(4-hydraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Coagulation factor X | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069297 (1-[3-[4-amino(amineimino)methylphenyl]-2-(2-naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine trypsin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50069301 (CHEMBL155311 | N-hydroxyethyl-N-cyclopentyl-3-(4-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against bovine thrombin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against tissue plasminogen activator | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against human plasmin | Bioorg Med Chem Lett 8: 631-4 (1999) BindingDB Entry DOI: 10.7270/Q2CR5SHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554464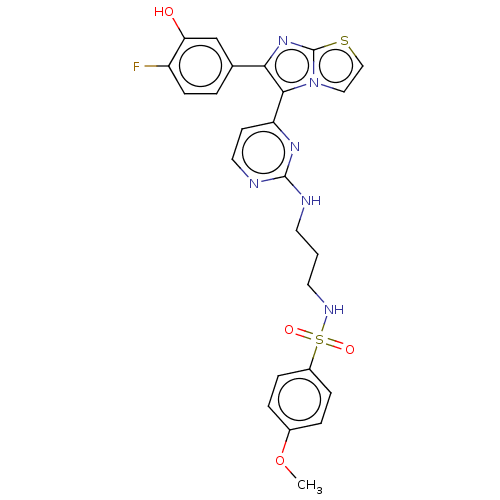 (US11332479, Compound 50III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50585092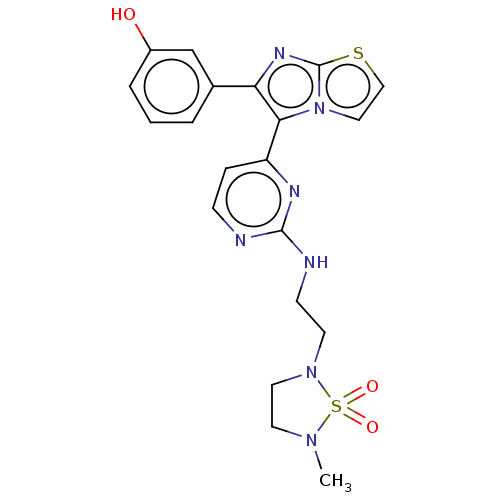 (CHEMBL5087577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50059889 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ROS1 by HotSpot assay relative to control | Bioorg Med Chem Lett 19: 4720-3 (2009) Article DOI: 10.1016/j.bmcl.2009.06.066 BindingDB Entry DOI: 10.7270/Q2Z89CG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554470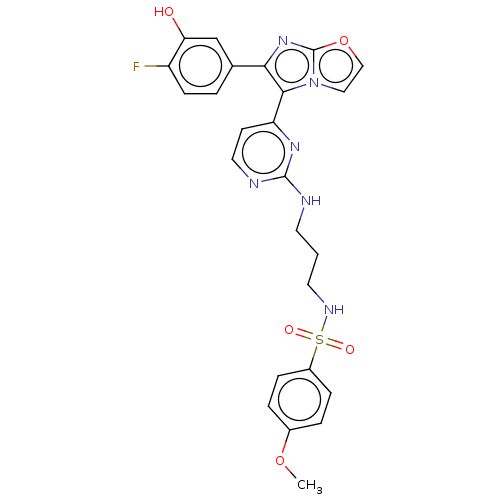 (US11332479, Compound 72IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554469 (US11332479, Compound 70IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554462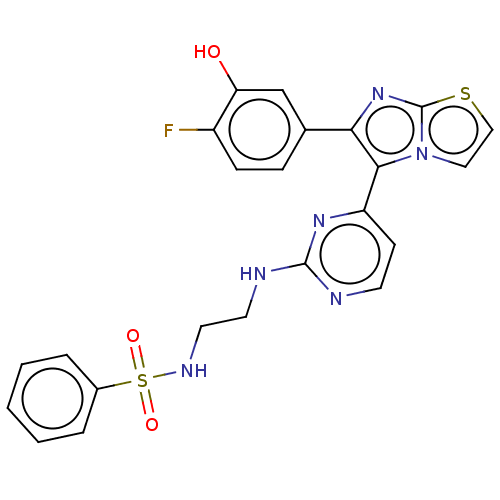 (US11332479, Compound 46III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554471 (US11332479, Compound 73IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554463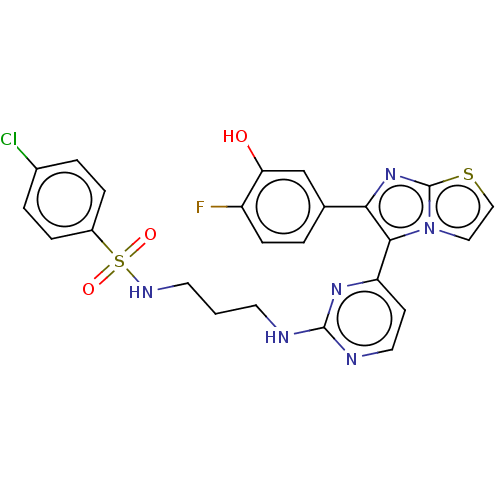 (US11332479, Compound 48III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554467 (US11332479, Compound 64IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554460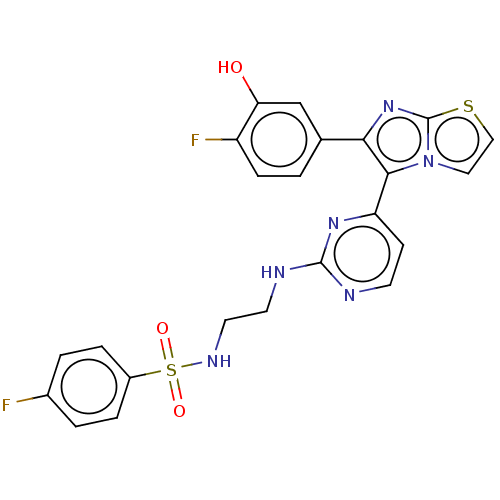 (US11332479, Compound 42III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554468 (US11332479, Compound 65IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50399540 (FORETINIB | US10464902, Foretinib | US10882853, Co...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of c-Met kinase (unknown origin) | Eur J Med Chem 90: 195-208 (2015) Article DOI: 10.1016/j.ejmech.2014.11.023 BindingDB Entry DOI: 10.7270/Q2M61MX6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50457446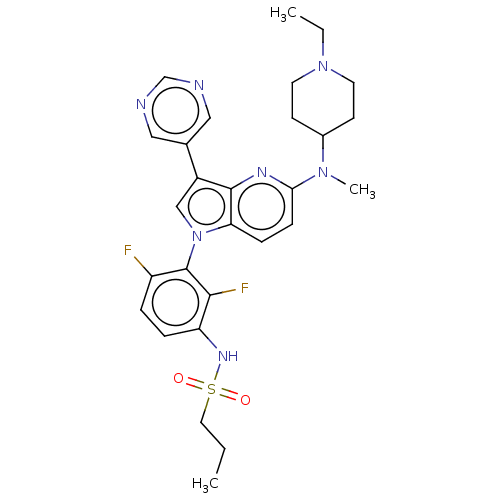 (CHEMBL4212692) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre (NRC ID: 60014618) Curated by ChEMBL | Assay Description Inhibition of BRAF (unknown origin) | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115493 BindingDB Entry DOI: 10.7270/Q2VX0M3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50506637 (CHEMBL4476226) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... | Bioorg Med Chem Lett 28: 3761-3765 (2018) Article DOI: 10.1016/j.bmcl.2018.10.013 BindingDB Entry DOI: 10.7270/Q23T9MHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50428286 (DABRAFENIB | GSK2118436A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF V600E mutant (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127478 BindingDB Entry DOI: 10.7270/Q2862M5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50506637 (CHEMBL4476226) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human AXL using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 28: 3761-3765 (2018) Article DOI: 10.1016/j.bmcl.2018.10.013 BindingDB Entry DOI: 10.7270/Q23T9MHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50296896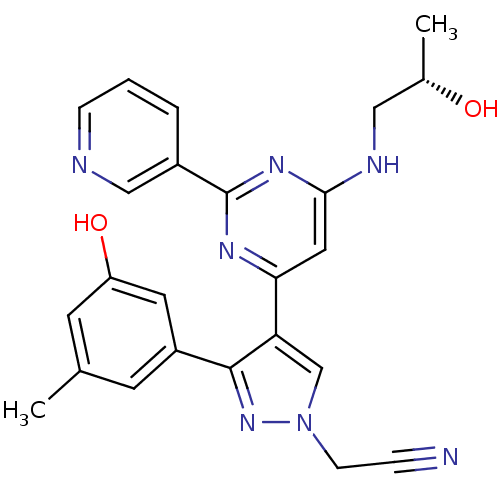 ((S)-2-(3-(3-hydroxy-5-methylphenyl)-4-(6-(2-hydrox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of ALK (unknown origin) incubated for 20 mins followed by [33P]ATP addition measured after 120 mins by HotSpot assay | Eur J Med Chem 90: 195-208 (2015) Article DOI: 10.1016/j.ejmech.2014.11.023 BindingDB Entry DOI: 10.7270/Q2M61MX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50062357 (AP26113 | CHEMBL3397300) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of ALK (unknown origin) | Eur J Med Chem 90: 195-208 (2015) Article DOI: 10.1016/j.ejmech.2014.11.023 BindingDB Entry DOI: 10.7270/Q2M61MX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50306682 ((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) | Eur J Med Chem 90: 195-208 (2015) Article DOI: 10.1016/j.ejmech.2014.11.023 BindingDB Entry DOI: 10.7270/Q2M61MX6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor [696-1022,L858R] (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50506648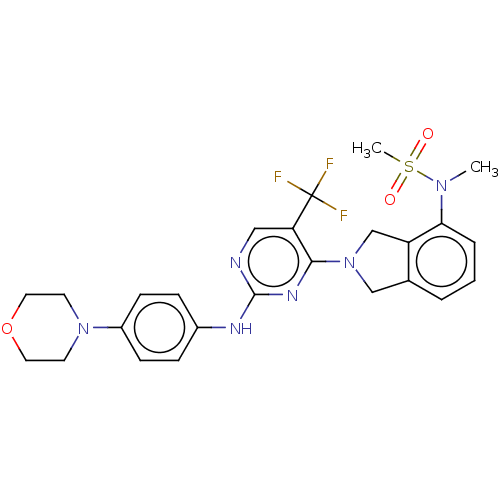 (CHEMBL4445940) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... | Bioorg Med Chem Lett 28: 3761-3765 (2018) Article DOI: 10.1016/j.bmcl.2018.10.013 BindingDB Entry DOI: 10.7270/Q23T9MHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435722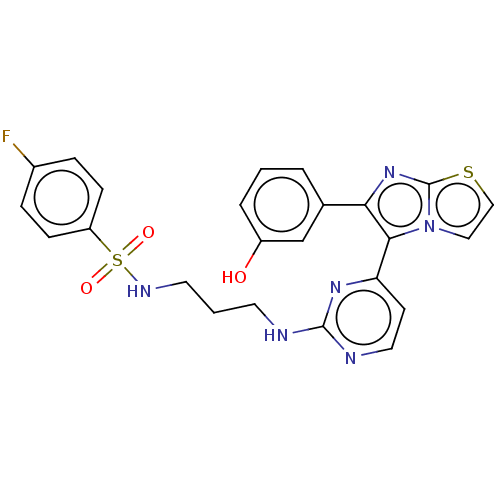 (US10570155, Compound 31III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554457 (US11332479, Compound 31II) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50585081 (CHEMBL5086749) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50587680 (CHEMBL5182271) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00533 BindingDB Entry DOI: 10.7270/Q23X8BM3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435721 (US10570155, Compound 25III | US11332479, Compound ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM435721 (US10570155, Compound 25III | US11332479, Compound ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50587677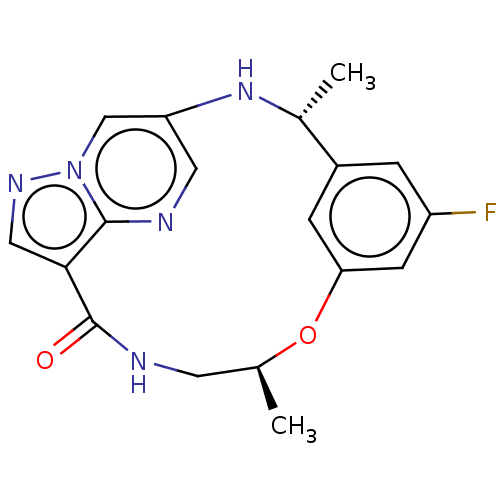 (CHEMBL5173501) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00533 BindingDB Entry DOI: 10.7270/Q23X8BM3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435721 (US10570155, Compound 25III | US11332479, Compound ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 566 total ) | Next | Last >> |