 Found 49 hits with Last Name = 'abate' and Initial = 'l'
Found 49 hits with Last Name = 'abate' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517320

(CHEMBL4568153)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)60-48(69)40-27-75-74-26-39(58-44(65)34(53)24-42(63)64)47(68)57-38(22-29-11-5-4-6-12-29)50(71)61(3)41(23-31-25-54-35-14-8-7-13-33(31)35)49(70)55-36(15-9-10-20-52)45(66)56-37(46(67)59-40)21-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,34,36-41,43,54,62H,9-10,15,20-24,26-27,52-53H2,1-3H3,(H,55,70)(H,56,66)(H,57,68)(H,58,65)(H,59,67)(H,60,69)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517327

(CHEMBL4472928)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](N(C)C(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C50H66N10O10S2/c1-28(2)42(50(69)70)59-47(66)40-26-71-72-27-41(60(4)49(68)29(3)52)48(67)57-38(22-30-12-6-5-7-13-30)44(63)56-39(24-32-25-53-35-15-9-8-14-34(32)35)46(65)54-36(16-10-11-21-51)43(62)55-37(45(64)58-40)23-31-17-19-33(61)20-18-31/h5-9,12-15,17-20,25,28-29,36-42,53,61H,10-11,16,21-24,26-27,51-52H2,1-4H3,(H,54,65)(H,55,62)(H,56,63)(H,57,67)(H,58,64)(H,59,66)(H,69,70)/t29-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517312
(CHEMBL4526244)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C50H66N10O10S2/c1-28(2)42(50(69)70)59-47(66)40-27-72-71-26-39(57-43(62)29(3)52)46(65)56-38(23-30-12-6-5-7-13-30)49(68)60(4)41(24-32-25-53-35-15-9-8-14-34(32)35)48(67)54-36(16-10-11-21-51)44(63)55-37(45(64)58-40)22-31-17-19-33(61)20-18-31/h5-9,12-15,17-20,25,28-29,36-42,53,61H,10-11,16,21-24,26-27,51-52H2,1-4H3,(H,54,67)(H,55,63)(H,56,65)(H,57,62)(H,58,64)(H,59,66)(H,69,70)/t29-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517325

(CHEMBL4590360)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](N(C)C(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)60-48(69)40-26-74-75-27-41(61(3)50(71)34(53)24-42(63)64)49(70)58-38(21-29-11-5-4-6-12-29)45(66)57-39(23-31-25-54-35-14-8-7-13-33(31)35)47(68)55-36(15-9-10-20-52)44(65)56-37(46(67)59-40)22-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,34,36-41,43,54,62H,9-10,15,20-24,26-27,52-53H2,1-3H3,(H,55,68)(H,56,65)(H,57,66)(H,58,70)(H,59,67)(H,60,69)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517315
(CHEMBL4454498)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1C)C(O)=O |r| Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)60-49(70)41-27-75-74-26-40(59-44(65)34(53)24-42(63)64)48(69)56-37(21-29-11-5-4-6-12-29)46(67)57-38(23-31-25-54-35-14-8-7-13-33(31)35)47(68)55-36(15-9-10-20-52)45(66)58-39(50(71)61(41)3)22-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,34,36-41,43,54,62H,9-10,15,20-24,26-27,52-53H2,1-3H3,(H,55,68)(H,56,69)(H,57,67)(H,58,66)(H,59,65)(H,60,70)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517316

(CHEMBL4462940)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1C)C(O)=O |r| Show InChI InChI=1S/C50H66N10O10S2/c1-28(2)42(50(69)70)59-48(67)41-27-72-71-26-40(58-43(62)29(3)52)47(66)55-37(22-30-12-6-5-7-13-30)45(64)56-38(24-32-25-53-35-15-9-8-14-34(32)35)46(65)54-36(16-10-11-21-51)44(63)57-39(49(68)60(41)4)23-31-17-19-33(61)20-18-31/h5-9,12-15,17-20,25,28-29,36-42,53,61H,10-11,16,21-24,26-27,51-52H2,1-4H3,(H,54,65)(H,55,66)(H,56,64)(H,57,63)(H,58,62)(H,59,67)(H,69,70)/t29-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50320463
(CHEMBL218994 | D[CFWKYC]V | H-Asp-Cys-Phe-Trp-Lys-...)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C50H64N10O12S2/c1-27(2)42(50(71)72)60-49(70)40-26-74-73-25-39(58-43(64)33(52)23-41(62)63)48(69)56-36(20-28-10-4-3-5-11-28)45(66)57-38(22-30-24-53-34-13-7-6-12-32(30)34)47(68)54-35(14-8-9-19-51)44(65)55-37(46(67)59-40)21-29-15-17-31(61)18-16-29/h3-7,10-13,15-18,24,27,33,35-40,42,53,61H,8-9,14,19-23,25-26,51-52H2,1-2H3,(H,54,68)(H,55,65)(H,56,69)(H,57,66)(H,58,64)(H,59,67)(H,60,70)(H,62,63)(H,71,72)/t33-,35-,36-,37-,38-,39-,40-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50445383
(CHEMBL3104471)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C49H64N10O10S2/c1-27(2)41(49(68)69)59-48(67)40-26-71-70-25-39(57-42(61)28(3)51)47(66)55-36(21-29-11-5-4-6-12-29)44(63)56-38(23-31-24-52-34-14-8-7-13-33(31)34)46(65)53-35(15-9-10-20-50)43(62)54-37(45(64)58-40)22-30-16-18-32(60)19-17-30/h4-8,11-14,16-19,24,27-28,35-41,52,60H,9-10,15,20-23,25-26,50-51H2,1-3H3,(H,53,65)(H,54,62)(H,55,66)(H,56,63)(H,57,61)(H,58,64)(H,59,67)(H,68,69)/t28-,35-,36-,37-,38-,39-,40-,41-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517314
(Urotensin-II)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C64H85N13O18S2/c1-33(2)52(64(94)95)75-61(91)48-32-97-96-31-47(73-59(89)46(29-51(82)83)72-62(92)49-17-11-25-77(49)63(93)53(34(3)78)76-54(84)40(66)22-23-50(80)81)60(90)70-43(26-35-12-5-4-6-13-35)56(86)71-45(28-37-30-67-41-15-8-7-14-39(37)41)58(88)68-42(16-9-10-24-65)55(85)69-44(57(87)74-48)27-36-18-20-38(79)21-19-36/h4-8,12-15,18-21,30,33-34,40,42-49,52-53,67,78-79H,9-11,16-17,22-29,31-32,65-66H2,1-3H3,(H,68,88)(H,69,85)(H,70,90)(H,71,86)(H,72,92)(H,73,89)(H,74,87)(H,75,91)(H,76,84)(H,80,81)(H,82,83)(H,94,95)/t34-,40+,42+,43+,44+,45+,46+,47+,48+,49+,52+,53+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517317

(CHEMBL4475783)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C65H87N13O18S2/c1-34(2)53(65(95)96)75-60(90)48-33-98-97-32-47(73-58(88)45(30-52(83)84)71-61(91)49-18-12-26-78(49)64(94)54(35(3)79)76-55(85)41(67)23-24-51(81)82)59(89)72-46(28-36-13-6-5-7-14-36)63(93)77(4)50(29-38-31-68-42-16-9-8-15-40(38)42)62(92)69-43(17-10-11-25-66)56(86)70-44(57(87)74-48)27-37-19-21-39(80)22-20-37/h5-9,13-16,19-22,31,34-35,41,43-50,53-54,68,79-80H,10-12,17-18,23-30,32-33,66-67H2,1-4H3,(H,69,92)(H,70,86)(H,71,91)(H,72,89)(H,73,88)(H,74,87)(H,75,90)(H,76,85)(H,81,82)(H,83,84)(H,95,96)/t35-,41+,43+,44+,45+,46+,47+,48+,49+,50+,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517323
(CHEMBL4474481)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1C)C(O)=O |r| Show InChI InChI=1S/C65H87N13O18S2/c1-34(2)53(65(95)96)75-62(92)50-33-98-97-32-48(74-59(89)46(30-52(83)84)72-61(91)49-18-12-26-78(49)64(94)54(35(3)79)76-55(85)41(67)23-24-51(81)82)60(90)70-44(27-36-13-6-5-7-14-36)57(87)71-45(29-38-31-68-42-16-9-8-15-40(38)42)58(88)69-43(17-10-11-25-66)56(86)73-47(63(93)77(50)4)28-37-19-21-39(80)22-20-37/h5-9,13-16,19-22,31,34-35,41,43-50,53-54,68,79-80H,10-12,17-18,23-30,32-33,66-67H2,1-4H3,(H,69,88)(H,70,90)(H,71,87)(H,72,91)(H,73,86)(H,74,89)(H,75,92)(H,76,85)(H,81,82)(H,83,84)(H,95,96)/t35-,41+,43+,44+,45+,46+,47+,48+,49+,50+,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517324
(CHEMBL4449844)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](N(C)C(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C65H87N13O18S2/c1-34(2)53(65(95)96)75-60(90)48-32-97-98-33-50(77(4)63(93)47(30-52(83)84)73-61(91)49-18-12-26-78(49)64(94)54(35(3)79)76-55(85)41(67)23-24-51(81)82)62(92)72-45(27-36-13-6-5-7-14-36)57(87)71-46(29-38-31-68-42-16-9-8-15-40(38)42)59(89)69-43(17-10-11-25-66)56(86)70-44(58(88)74-48)28-37-19-21-39(80)22-20-37/h5-9,13-16,19-22,31,34-35,41,43-50,53-54,68,79-80H,10-12,17-18,23-30,32-33,66-67H2,1-4H3,(H,69,89)(H,70,86)(H,71,87)(H,72,92)(H,73,91)(H,74,88)(H,75,90)(H,76,85)(H,81,82)(H,83,84)(H,95,96)/t35-,41+,43+,44+,45+,46+,47+,48+,49+,50+,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517321

(CHEMBL4568539)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N(C)[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)60-48(69)39-26-74-75-27-40(59-44(65)34(53)24-42(63)64)50(71)61(3)41(22-29-11-5-4-6-12-29)49(70)57-38(23-31-25-54-35-14-8-7-13-33(31)35)47(68)55-36(15-9-10-20-52)45(66)56-37(46(67)58-39)21-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,34,36-41,43,54,62H,9-10,15,20-24,26-27,52-53H2,1-3H3,(H,55,68)(H,56,66)(H,57,70)(H,58,67)(H,59,65)(H,60,69)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517322
(CHEMBL4447811)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N(C)[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C50H66N10O10S2/c1-28(2)42(50(69)70)59-47(66)39-26-71-72-27-40(58-43(62)29(3)52)49(68)60(4)41(23-30-12-6-5-7-13-30)48(67)56-38(24-32-25-53-35-15-9-8-14-34(32)35)46(65)54-36(16-10-11-21-51)44(63)55-37(45(64)57-39)22-31-17-19-33(61)20-18-31/h5-9,12-15,17-20,25,28-29,36-42,53,61H,10-11,16,21-24,26-27,51-52H2,1-4H3,(H,54,65)(H,55,63)(H,56,67)(H,57,64)(H,58,62)(H,59,66)(H,69,70)/t29-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517318
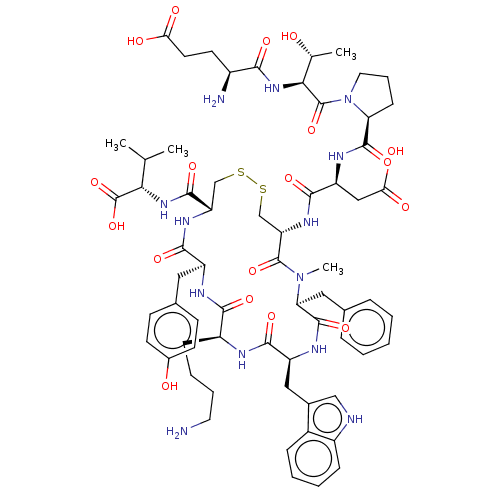
(CHEMBL4467241)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N(C)[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C65H87N13O18S2/c1-34(2)53(65(95)96)75-60(90)47-32-97-98-33-48(74-59(89)46(30-52(83)84)72-61(91)49-18-12-26-78(49)64(94)54(35(3)79)76-55(85)41(67)23-24-51(81)82)63(93)77(4)50(28-36-13-6-5-7-14-36)62(92)71-45(29-38-31-68-42-16-9-8-15-40(38)42)58(88)69-43(17-10-11-25-66)56(86)70-44(57(87)73-47)27-37-19-21-39(80)22-20-37/h5-9,13-16,19-22,31,34-35,41,43-50,53-54,68,79-80H,10-12,17-18,23-30,32-33,66-67H2,1-4H3,(H,69,88)(H,70,86)(H,71,92)(H,72,91)(H,73,87)(H,74,89)(H,75,90)(H,76,85)(H,81,82)(H,83,84)(H,95,96)/t35-,41+,43+,44+,45+,46+,47+,48+,49+,50+,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517313

(CHEMBL4483461)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N(C)[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C50H66N10O10S2/c1-28(2)42(50(69)70)59-47(66)40-27-72-71-26-39(57-43(62)29(3)52)46(65)54-36(22-30-12-6-5-7-13-30)44(63)56-38(24-32-25-53-35-15-9-8-14-34(32)35)49(68)60(4)41(16-10-11-21-51)48(67)55-37(45(64)58-40)23-31-17-19-33(61)20-18-31/h5-9,12-15,17-20,25,28-29,36-42,53,61H,10-11,16,21-24,26-27,51-52H2,1-4H3,(H,54,65)(H,55,67)(H,56,63)(H,57,62)(H,58,64)(H,59,66)(H,69,70)/t29-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517326

(CHEMBL4549818)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N(C)[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C65H87N13O18S2/c1-34(2)53(65(95)96)75-60(90)48-33-98-97-32-47(73-58(88)45(30-52(83)84)71-62(92)50-18-12-26-78(50)64(94)54(35(3)79)76-55(85)41(67)23-24-51(81)82)59(89)69-43(27-36-13-6-5-7-14-36)56(86)72-46(29-38-31-68-42-16-9-8-15-40(38)42)63(93)77(4)49(17-10-11-25-66)61(91)70-44(57(87)74-48)28-37-19-21-39(80)22-20-37/h5-9,13-16,19-22,31,34-35,41,43-50,53-54,68,79-80H,10-12,17-18,23-30,32-33,66-67H2,1-4H3,(H,69,89)(H,70,91)(H,71,92)(H,72,86)(H,73,88)(H,74,87)(H,75,90)(H,76,85)(H,81,82)(H,83,84)(H,95,96)/t35-,41+,43+,44+,45+,46+,47+,48+,49+,50+,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517330
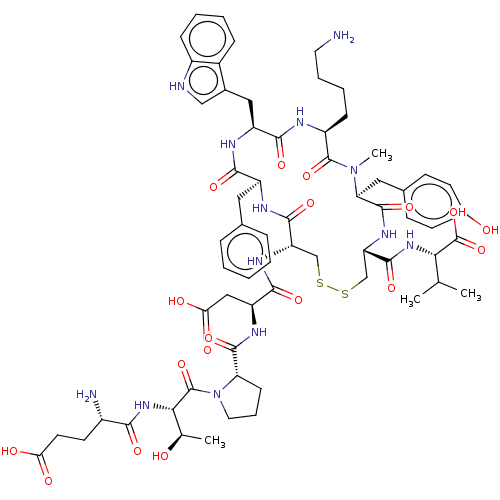
(CHEMBL4455086)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N(C)[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C65H87N13O18S2/c1-34(2)53(65(95)96)75-60(90)48-33-98-97-32-47(73-58(88)46(30-52(83)84)72-61(91)49-18-12-26-78(49)64(94)54(35(3)79)76-55(85)41(67)23-24-51(81)82)59(89)70-44(27-36-13-6-5-7-14-36)56(86)71-45(29-38-31-68-42-16-9-8-15-40(38)42)57(87)69-43(17-10-11-25-66)63(93)77(4)50(62(92)74-48)28-37-19-21-39(80)22-20-37/h5-9,13-16,19-22,31,34-35,41,43-50,53-54,68,79-80H,10-12,17-18,23-30,32-33,66-67H2,1-4H3,(H,69,87)(H,70,89)(H,71,86)(H,72,91)(H,73,88)(H,74,92)(H,75,90)(H,76,85)(H,81,82)(H,83,84)(H,95,96)/t35-,41+,43+,44+,45+,46+,47+,48+,49+,50+,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517329

(CHEMBL4565187)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N(C)[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C50H66N10O10S2/c1-28(2)42(50(69)70)59-47(66)40-27-72-71-26-39(57-43(62)29(3)52)46(65)55-37(22-30-12-6-5-7-13-30)44(63)56-38(24-32-25-53-35-15-9-8-14-34(32)35)45(64)54-36(16-10-11-21-51)49(68)60(4)41(48(67)58-40)23-31-17-19-33(61)20-18-31/h5-9,12-15,17-20,25,28-29,36-42,53,61H,10-11,16,21-24,26-27,51-52H2,1-4H3,(H,54,64)(H,55,65)(H,56,63)(H,57,62)(H,58,67)(H,59,66)(H,69,70)/t29-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 5.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517319
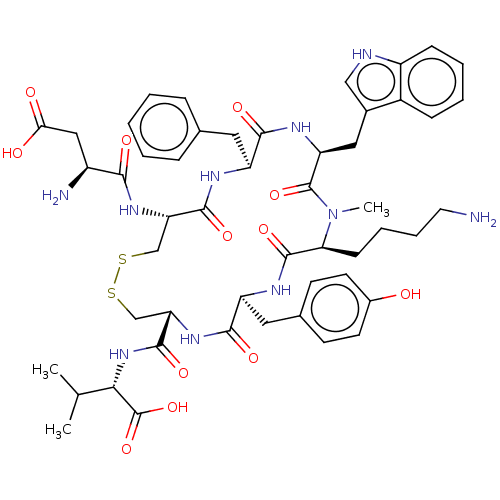
(CHEMBL4544618)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N(C)[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)60-48(69)40-27-75-74-26-39(58-44(65)34(53)24-42(63)64)47(68)55-36(21-29-11-5-4-6-12-29)45(66)57-38(23-31-25-54-35-14-8-7-13-33(31)35)50(71)61(3)41(15-9-10-20-52)49(70)56-37(46(67)59-40)22-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,34,36-41,43,54,62H,9-10,15,20-24,26-27,52-53H2,1-3H3,(H,55,68)(H,56,70)(H,57,66)(H,58,65)(H,59,67)(H,60,69)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517328

(CHEMBL4540501)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N(C)[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)60-48(69)40-27-75-74-26-39(58-44(65)34(53)24-42(63)64)47(68)56-37(21-29-11-5-4-6-12-29)45(66)57-38(23-31-25-54-35-14-8-7-13-33(31)35)46(67)55-36(15-9-10-20-52)50(71)61(3)41(49(70)59-40)22-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,34,36-41,43,54,62H,9-10,15,20-24,26-27,52-53H2,1-3H3,(H,55,67)(H,56,68)(H,57,66)(H,58,65)(H,59,70)(H,60,69)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-URP or human [125I[-urotensin-2 from human urotensin 2 receptor expressed in HEK293 or CHOK1 cells after 2 hrs by gamma-counti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus 2) | BDBM50603527
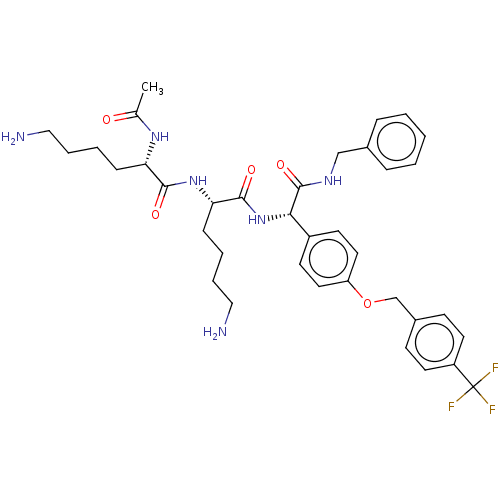
(CHEMBL5177478)Show SMILES CC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(=O)NCc1ccccc1)c1ccc(OCc2ccc(cc2)C(F)(F)F)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116631
BindingDB Entry DOI: 10.7270/Q2GF0ZKN |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM50603527

(CHEMBL5177478)Show SMILES CC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(=O)NCc1ccccc1)c1ccc(OCc2ccc(cc2)C(F)(F)F)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116631
BindingDB Entry DOI: 10.7270/Q2GF0ZKN |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50320463

(CHEMBL218994 | D[CFWKYC]V | H-Asp-Cys-Phe-Trp-Lys-...)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C50H64N10O12S2/c1-27(2)42(50(71)72)60-49(70)40-26-74-73-25-39(58-43(64)33(52)23-41(62)63)48(69)56-36(20-28-10-4-3-5-11-28)45(66)57-38(22-30-24-53-34-13-7-6-12-32(30)34)47(68)54-35(14-8-9-19-51)44(65)55-37(46(67)59-40)21-29-15-17-31(61)18-16-29/h3-7,10-13,15-18,24,27,33,35-40,42,53,61H,8-9,14,19-23,25-26,51-52H2,1-2H3,(H,54,68)(H,55,65)(H,56,69)(H,57,66)(H,58,64)(H,59,67)(H,60,70)(H,62,63)(H,71,72)/t33-,35-,36-,37-,38-,39-,40-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517317

(CHEMBL4475783)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C65H87N13O18S2/c1-34(2)53(65(95)96)75-60(90)48-33-98-97-32-47(73-58(88)45(30-52(83)84)71-61(91)49-18-12-26-78(49)64(94)54(35(3)79)76-55(85)41(67)23-24-51(81)82)59(89)72-46(28-36-13-6-5-7-14-36)63(93)77(4)50(29-38-31-68-42-16-9-8-15-40(38)42)62(92)69-43(17-10-11-25-66)56(86)70-44(57(87)74-48)27-37-19-21-39(80)22-20-37/h5-9,13-16,19-22,31,34-35,41,43-50,53-54,68,79-80H,10-12,17-18,23-30,32-33,66-67H2,1-4H3,(H,69,92)(H,70,86)(H,71,91)(H,72,89)(H,73,88)(H,74,87)(H,75,90)(H,76,85)(H,81,82)(H,83,84)(H,95,96)/t35-,41+,43+,44+,45+,46+,47+,48+,49+,50+,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor (unknown origin) expressed in HEK293 cells co-expressing Gq-polycistronic BRET biosensor assessed as inducti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517321

(CHEMBL4568539)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N(C)[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)60-48(69)39-26-74-75-27-40(59-44(65)34(53)24-42(63)64)50(71)61(3)41(22-29-11-5-4-6-12-29)49(70)57-38(23-31-25-54-35-14-8-7-13-33(31)35)47(68)55-36(15-9-10-20-52)45(66)56-37(46(67)58-39)21-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,34,36-41,43,54,62H,9-10,15,20-24,26-27,52-53H2,1-3H3,(H,55,68)(H,56,66)(H,57,70)(H,58,67)(H,59,65)(H,60,69)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor (unknown origin) expressed in HEK293 cells co-expressing Gq-polycistronic BRET biosensor assessed as inducti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50517314

(Urotensin-II)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C64H85N13O18S2/c1-33(2)52(64(94)95)75-61(91)48-32-97-96-31-47(73-59(89)46(29-51(82)83)72-62(92)49-17-11-25-77(49)63(93)53(34(3)78)76-54(84)40(66)22-23-50(80)81)60(90)70-43(26-35-12-5-4-6-13-35)56(86)71-45(28-37-30-67-41-15-8-7-14-39(37)41)58(88)68-42(16-9-10-24-65)55(85)69-44(57(87)74-48)27-36-18-20-38(79)21-19-36/h4-8,12-15,18-21,30,33-34,40,42-49,52-53,67,78-79H,9-11,16-17,22-29,31-32,65-66H2,1-3H3,(H,68,88)(H,69,85)(H,70,90)(H,71,86)(H,72,92)(H,73,89)(H,74,87)(H,75,91)(H,76,84)(H,80,81)(H,82,83)(H,94,95)/t34-,40+,42+,43+,44+,45+,46+,47+,48+,49+,52+,53+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50517323

(CHEMBL4474481)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1C)C(O)=O |r| Show InChI InChI=1S/C65H87N13O18S2/c1-34(2)53(65(95)96)75-62(92)50-33-98-97-32-48(74-59(89)46(30-52(83)84)72-61(91)49-18-12-26-78(49)64(94)54(35(3)79)76-55(85)41(67)23-24-51(81)82)60(90)70-44(27-36-13-6-5-7-14-36)57(87)71-45(29-38-31-68-42-16-9-8-15-40(38)42)58(88)69-43(17-10-11-25-66)56(86)73-47(63(93)77(50)4)28-37-19-21-39(80)22-20-37/h5-9,13-16,19-22,31,34-35,41,43-50,53-54,68,79-80H,10-12,17-18,23-30,32-33,66-67H2,1-4H3,(H,69,88)(H,70,90)(H,71,87)(H,72,91)(H,73,86)(H,74,89)(H,75,92)(H,76,85)(H,81,82)(H,83,84)(H,95,96)/t35-,41+,43+,44+,45+,46+,47+,48+,49+,50+,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517318

(CHEMBL4467241)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N(C)[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C65H87N13O18S2/c1-34(2)53(65(95)96)75-60(90)47-32-97-98-33-48(74-59(89)46(30-52(83)84)72-61(91)49-18-12-26-78(49)64(94)54(35(3)79)76-55(85)41(67)23-24-51(81)82)63(93)77(4)50(28-36-13-6-5-7-14-36)62(92)71-45(29-38-31-68-42-16-9-8-15-40(38)42)58(88)69-43(17-10-11-25-66)56(86)70-44(57(87)73-47)27-37-19-21-39(80)22-20-37/h5-9,13-16,19-22,31,34-35,41,43-50,53-54,68,79-80H,10-12,17-18,23-30,32-33,66-67H2,1-4H3,(H,69,88)(H,70,86)(H,71,92)(H,72,91)(H,73,87)(H,74,89)(H,75,90)(H,76,85)(H,81,82)(H,83,84)(H,95,96)/t35-,41+,43+,44+,45+,46+,47+,48+,49+,50+,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor (unknown origin) expressed in HEK293 cells co-expressing Gq-polycistronic BRET biosensor assessed as inducti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50445383

(CHEMBL3104471)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C49H64N10O10S2/c1-27(2)41(49(68)69)59-48(67)40-26-71-70-25-39(57-42(61)28(3)51)47(66)55-36(21-29-11-5-4-6-12-29)44(63)56-38(23-31-24-52-34-14-8-7-13-33(31)34)46(65)53-35(15-9-10-20-50)43(62)54-37(45(64)58-40)22-30-16-18-32(60)19-17-30/h4-8,11-14,16-19,24,27-28,35-41,52,60H,9-10,15,20-23,25-26,50-51H2,1-3H3,(H,53,65)(H,54,62)(H,55,66)(H,56,63)(H,57,61)(H,58,64)(H,59,67)(H,68,69)/t28-,35-,36-,37-,38-,39-,40-,41-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor (unknown origin) expressed in HEK293 cells co-expressing Gq-polycistronic BRET biosensor assessed as inducti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517320

(CHEMBL4568153)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)60-48(69)40-27-75-74-26-39(58-44(65)34(53)24-42(63)64)47(68)57-38(22-29-11-5-4-6-12-29)50(71)61(3)41(23-31-25-54-35-14-8-7-13-33(31)35)49(70)55-36(15-9-10-20-52)45(66)56-37(46(67)59-40)21-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,34,36-41,43,54,62H,9-10,15,20-24,26-27,52-53H2,1-3H3,(H,55,70)(H,56,66)(H,57,68)(H,58,65)(H,59,67)(H,60,69)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor (unknown origin) expressed in HEK293 cells co-expressing Gq-polycistronic BRET biosensor assessed as inducti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50517324

(CHEMBL4449844)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](N(C)C(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C65H87N13O18S2/c1-34(2)53(65(95)96)75-60(90)48-32-97-98-33-50(77(4)63(93)47(30-52(83)84)73-61(91)49-18-12-26-78(49)64(94)54(35(3)79)76-55(85)41(67)23-24-51(81)82)62(92)72-45(27-36-13-6-5-7-14-36)57(87)71-46(29-38-31-68-42-16-9-8-15-40(38)42)59(89)69-43(17-10-11-25-66)56(86)70-44(58(88)74-48)28-37-19-21-39(80)22-20-37/h5-9,13-16,19-22,31,34-35,41,43-50,53-54,68,79-80H,10-12,17-18,23-30,32-33,66-67H2,1-4H3,(H,69,89)(H,70,86)(H,71,87)(H,72,92)(H,73,91)(H,74,88)(H,75,90)(H,76,85)(H,81,82)(H,83,84)(H,95,96)/t35-,41+,43+,44+,45+,46+,47+,48+,49+,50+,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50517327

(CHEMBL4472928)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](N(C)C(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C50H66N10O10S2/c1-28(2)42(50(69)70)59-47(66)40-26-71-72-27-41(60(4)49(68)29(3)52)48(67)57-38(22-30-12-6-5-7-13-30)44(63)56-39(24-32-25-53-35-15-9-8-14-34(32)35)46(65)54-36(16-10-11-21-51)43(62)55-37(45(64)58-40)23-31-17-19-33(61)20-18-31/h5-9,12-15,17-20,25,28-29,36-42,53,61H,10-11,16,21-24,26-27,51-52H2,1-4H3,(H,54,65)(H,55,62)(H,56,63)(H,57,67)(H,58,64)(H,59,66)(H,69,70)/t29-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50517320

(CHEMBL4568153)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)60-48(69)40-27-75-74-26-39(58-44(65)34(53)24-42(63)64)47(68)57-38(22-29-11-5-4-6-12-29)50(71)61(3)41(23-31-25-54-35-14-8-7-13-33(31)35)49(70)55-36(15-9-10-20-52)45(66)56-37(46(67)59-40)21-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,34,36-41,43,54,62H,9-10,15,20-24,26-27,52-53H2,1-3H3,(H,55,70)(H,56,66)(H,57,68)(H,58,65)(H,59,67)(H,60,69)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517322

(CHEMBL4447811)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N(C)[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C50H66N10O10S2/c1-28(2)42(50(69)70)59-47(66)39-26-71-72-27-40(58-43(62)29(3)52)49(68)60(4)41(23-30-12-6-5-7-13-30)48(67)56-38(24-32-25-53-35-15-9-8-14-34(32)35)46(65)54-36(16-10-11-21-51)44(63)55-37(45(64)57-39)22-31-17-19-33(61)20-18-31/h5-9,12-15,17-20,25,28-29,36-42,53,61H,10-11,16,21-24,26-27,51-52H2,1-4H3,(H,54,65)(H,55,63)(H,56,67)(H,57,64)(H,58,62)(H,59,66)(H,69,70)/t29-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 105 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor (unknown origin) expressed in HEK293 cells co-expressing Gq-polycistronic BRET biosensor assessed as inducti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50517321

(CHEMBL4568539)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N(C)[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)60-48(69)39-26-74-75-27-40(59-44(65)34(53)24-42(63)64)50(71)61(3)41(22-29-11-5-4-6-12-29)49(70)57-38(23-31-25-54-35-14-8-7-13-33(31)35)47(68)55-36(15-9-10-20-52)45(66)56-37(46(67)58-39)21-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,34,36-41,43,54,62H,9-10,15,20-24,26-27,52-53H2,1-3H3,(H,55,68)(H,56,66)(H,57,70)(H,58,67)(H,59,65)(H,60,69)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 537 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517315

(CHEMBL4454498)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1C)C(O)=O |r| Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)60-49(70)41-27-75-74-26-40(59-44(65)34(53)24-42(63)64)48(69)56-37(21-29-11-5-4-6-12-29)46(67)57-38(23-31-25-54-35-14-8-7-13-33(31)35)47(68)55-36(15-9-10-20-52)45(66)58-39(50(71)61(41)3)22-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,34,36-41,43,54,62H,9-10,15,20-24,26-27,52-53H2,1-3H3,(H,55,68)(H,56,69)(H,57,67)(H,58,66)(H,59,65)(H,60,70)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor (unknown origin) expressed in HEK293 cells co-expressing Gq-polycistronic BRET biosensor assessed as inducti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50517325

(CHEMBL4590360)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](N(C)C(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)60-48(69)40-26-74-75-27-41(61(3)50(71)34(53)24-42(63)64)49(70)58-38(21-29-11-5-4-6-12-29)45(66)57-39(23-31-25-54-35-14-8-7-13-33(31)35)47(68)55-36(15-9-10-20-52)44(65)56-37(46(67)59-40)22-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,34,36-41,43,54,62H,9-10,15,20-24,26-27,52-53H2,1-3H3,(H,55,68)(H,56,65)(H,57,66)(H,58,70)(H,59,67)(H,60,69)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50320463

(CHEMBL218994 | D[CFWKYC]V | H-Asp-Cys-Phe-Trp-Lys-...)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O Show InChI InChI=1S/C50H64N10O12S2/c1-27(2)42(50(71)72)60-49(70)40-26-74-73-25-39(58-43(64)33(52)23-41(62)63)48(69)56-36(20-28-10-4-3-5-11-28)45(66)57-38(22-30-24-53-34-13-7-6-12-32(30)34)47(68)54-35(14-8-9-19-51)44(65)55-37(46(67)59-40)21-29-15-17-31(61)18-16-29/h3-7,10-13,15-18,24,27,33,35-40,42,53,61H,8-9,14,19-23,25-26,51-52H2,1-2H3,(H,54,68)(H,55,65)(H,56,69)(H,57,66)(H,58,64)(H,59,67)(H,60,70)(H,62,63)(H,71,72)/t33-,35-,36-,37-,38-,39-,40-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor (unknown origin) expressed in HEK293 cells co-expressing Gq-polycistronic BRET biosensor assessed as inducti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517323

(CHEMBL4474481)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1C)C(O)=O |r| Show InChI InChI=1S/C65H87N13O18S2/c1-34(2)53(65(95)96)75-62(92)50-33-98-97-32-48(74-59(89)46(30-52(83)84)72-61(91)49-18-12-26-78(49)64(94)54(35(3)79)76-55(85)41(67)23-24-51(81)82)60(90)70-44(27-36-13-6-5-7-14-36)57(87)71-45(29-38-31-68-42-16-9-8-15-40(38)42)58(88)69-43(17-10-11-25-66)56(86)73-47(63(93)77(50)4)28-37-19-21-39(80)22-20-37/h5-9,13-16,19-22,31,34-35,41,43-50,53-54,68,79-80H,10-12,17-18,23-30,32-33,66-67H2,1-4H3,(H,69,88)(H,70,90)(H,71,87)(H,72,91)(H,73,86)(H,74,89)(H,75,92)(H,76,85)(H,81,82)(H,83,84)(H,95,96)/t35-,41+,43+,44+,45+,46+,47+,48+,49+,50+,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor (unknown origin) expressed in HEK293 cells co-expressing Gq-polycistronic BRET biosensor assessed as inducti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517316

(CHEMBL4462940)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1C)C(O)=O |r| Show InChI InChI=1S/C50H66N10O10S2/c1-28(2)42(50(69)70)59-48(67)41-27-72-71-26-40(58-43(62)29(3)52)47(66)55-37(22-30-12-6-5-7-13-30)45(64)56-38(24-32-25-53-35-15-9-8-14-34(32)35)46(65)54-36(16-10-11-21-51)44(63)57-39(49(68)60(41)4)23-31-17-19-33(61)20-18-31/h5-9,12-15,17-20,25,28-29,36-42,53,61H,10-11,16,21-24,26-27,51-52H2,1-4H3,(H,54,65)(H,55,66)(H,56,64)(H,57,63)(H,58,62)(H,59,67)(H,69,70)/t29-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor (unknown origin) expressed in HEK293 cells co-expressing Gq-polycistronic BRET biosensor assessed as inducti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50445383

(CHEMBL3104471)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C49H64N10O10S2/c1-27(2)41(49(68)69)59-48(67)40-26-71-70-25-39(57-42(61)28(3)51)47(66)55-36(21-29-11-5-4-6-12-29)44(63)56-38(23-31-24-52-34-14-8-7-13-33(31)34)46(65)53-35(15-9-10-20-50)43(62)54-37(45(64)58-40)22-30-16-18-32(60)19-17-30/h4-8,11-14,16-19,24,27-28,35-41,52,60H,9-10,15,20-23,25-26,50-51H2,1-3H3,(H,53,65)(H,54,62)(H,55,66)(H,56,63)(H,57,61)(H,58,64)(H,59,67)(H,68,69)/t28-,35-,36-,37-,38-,39-,40-,41-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50517318

(CHEMBL4467241)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N(C)[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C65H87N13O18S2/c1-34(2)53(65(95)96)75-60(90)47-32-97-98-33-48(74-59(89)46(30-52(83)84)72-61(91)49-18-12-26-78(49)64(94)54(35(3)79)76-55(85)41(67)23-24-51(81)82)63(93)77(4)50(28-36-13-6-5-7-14-36)62(92)71-45(29-38-31-68-42-16-9-8-15-40(38)42)58(88)69-43(17-10-11-25-66)56(86)70-44(57(87)73-47)27-37-19-21-39(80)22-20-37/h5-9,13-16,19-22,31,34-35,41,43-50,53-54,68,79-80H,10-12,17-18,23-30,32-33,66-67H2,1-4H3,(H,69,88)(H,70,86)(H,71,92)(H,72,91)(H,73,87)(H,74,89)(H,75,90)(H,76,85)(H,81,82)(H,83,84)(H,95,96)/t35-,41+,43+,44+,45+,46+,47+,48+,49+,50+,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50517317

(CHEMBL4475783)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C65H87N13O18S2/c1-34(2)53(65(95)96)75-60(90)48-33-98-97-32-47(73-58(88)45(30-52(83)84)71-61(91)49-18-12-26-78(49)64(94)54(35(3)79)76-55(85)41(67)23-24-51(81)82)59(89)72-46(28-36-13-6-5-7-14-36)63(93)77(4)50(29-38-31-68-42-16-9-8-15-40(38)42)62(92)69-43(17-10-11-25-66)56(86)70-44(57(87)74-48)27-37-19-21-39(80)22-20-37/h5-9,13-16,19-22,31,34-35,41,43-50,53-54,68,79-80H,10-12,17-18,23-30,32-33,66-67H2,1-4H3,(H,69,92)(H,70,86)(H,71,91)(H,72,89)(H,73,88)(H,74,87)(H,75,90)(H,76,85)(H,81,82)(H,83,84)(H,95,96)/t35-,41+,43+,44+,45+,46+,47+,48+,49+,50+,53+,54+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50517316

(CHEMBL4462940)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1C)C(O)=O |r| Show InChI InChI=1S/C50H66N10O10S2/c1-28(2)42(50(69)70)59-48(67)41-27-72-71-26-40(58-43(62)29(3)52)47(66)55-37(22-30-12-6-5-7-13-30)45(64)56-38(24-32-25-53-35-15-9-8-14-34(32)35)46(65)54-36(16-10-11-21-51)44(63)57-39(49(68)60(41)4)23-31-17-19-33(61)20-18-31/h5-9,12-15,17-20,25,28-29,36-42,53,61H,10-11,16,21-24,26-27,51-52H2,1-4H3,(H,54,65)(H,55,66)(H,56,64)(H,57,63)(H,58,62)(H,59,67)(H,69,70)/t29-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50517315

(CHEMBL4454498)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1C)C(O)=O |r| Show InChI InChI=1S/C51H66N10O12S2/c1-28(2)43(51(72)73)60-49(70)41-27-75-74-26-40(59-44(65)34(53)24-42(63)64)48(69)56-37(21-29-11-5-4-6-12-29)46(67)57-38(23-31-25-54-35-14-8-7-13-33(31)35)47(68)55-36(15-9-10-20-52)45(66)58-39(50(71)61(41)3)22-30-16-18-32(62)19-17-30/h4-8,11-14,16-19,25,28,34,36-41,43,54,62H,9-10,15,20-24,26-27,52-53H2,1-3H3,(H,55,68)(H,56,69)(H,57,67)(H,58,66)(H,59,65)(H,60,70)(H,63,64)(H,72,73)/t34-,36-,37-,38-,39-,40-,41-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517314

(Urotensin-II)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C64H85N13O18S2/c1-33(2)52(64(94)95)75-61(91)48-32-97-96-31-47(73-59(89)46(29-51(82)83)72-62(92)49-17-11-25-77(49)63(93)53(34(3)78)76-54(84)40(66)22-23-50(80)81)60(90)70-43(26-35-12-5-4-6-13-35)56(86)71-45(28-37-30-67-41-15-8-7-14-39(37)41)58(88)68-42(16-9-10-24-65)55(85)69-44(57(87)74-48)27-36-18-20-38(79)21-19-36/h4-8,12-15,18-21,30,33-34,40,42-49,52-53,67,78-79H,9-11,16-17,22-29,31-32,65-66H2,1-3H3,(H,68,88)(H,69,85)(H,70,90)(H,71,86)(H,72,92)(H,73,89)(H,74,87)(H,75,91)(H,76,84)(H,80,81)(H,82,83)(H,94,95)/t34-,40+,42+,43+,44+,45+,46+,47+,48+,49+,52+,53+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor (unknown origin) expressed in HEK293 cells co-expressing Gq-polycistronic BRET biosensor assessed as inducti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50517312

(CHEMBL4526244)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C50H66N10O10S2/c1-28(2)42(50(69)70)59-47(66)40-27-72-71-26-39(57-43(62)29(3)52)46(65)56-38(23-30-12-6-5-7-13-30)49(68)60(4)41(24-32-25-53-35-15-9-8-14-34(32)35)48(67)54-36(16-10-11-21-51)44(63)55-37(45(64)58-40)22-31-17-19-33(61)20-18-31/h5-9,12-15,17-20,25,28-29,36-42,53,61H,10-11,16,21-24,26-27,51-52H2,1-4H3,(H,54,67)(H,55,63)(H,56,65)(H,57,62)(H,58,64)(H,59,66)(H,69,70)/t29-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor (unknown origin) expressed in HEK293 cells co-expressing Gq-polycistronic BRET biosensor assessed as inducti... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50517312

(CHEMBL4526244)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](C)N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N(C)[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(O)=O |r| Show InChI InChI=1S/C50H66N10O10S2/c1-28(2)42(50(69)70)59-47(66)40-27-72-71-26-39(57-43(62)29(3)52)46(65)56-38(23-30-12-6-5-7-13-30)49(68)60(4)41(24-32-25-53-35-15-9-8-14-34(32)35)48(67)54-36(16-10-11-21-51)44(63)55-37(45(64)58-40)22-31-17-19-33(61)20-18-31/h5-9,12-15,17-20,25,28-29,36-42,53,61H,10-11,16,21-24,26-27,51-52H2,1-4H3,(H,54,67)(H,55,63)(H,56,65)(H,57,62)(H,58,64)(H,59,66)(H,69,70)/t29-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 83 | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at urotensin 2 receptor in Sprague-Dawley rat thoracic aortic ring assessed as induction of contraction relative to KCl-induced cont... |
J Med Chem 62: 1455-1467 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01601
BindingDB Entry DOI: 10.7270/Q2PV6PRV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
















































