 Found 197 hits with Last Name = 'hwang' and Initial = 'l'
Found 197 hits with Last Name = 'hwang' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592246

(T3-CLK)Show SMILES CN1CCN(CC1)C(=O)C(C)(C)c1ccc(cc1)C(=O)Nc1cn2cc(ccc2n1)-c1ccncc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50260529
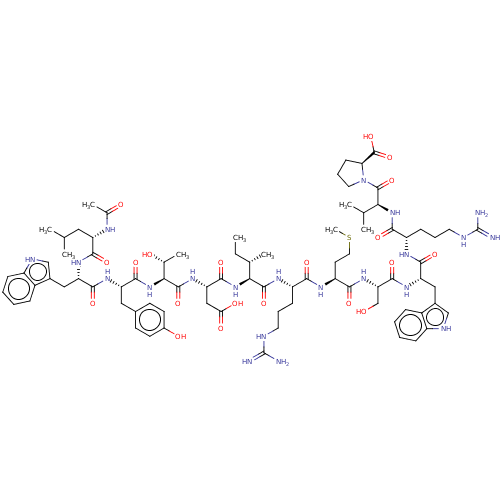
(CHEMBL4077626)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C83H121N21O20S/c1-10-44(6)67(102-76(118)62(38-65(109)110)99-79(121)68(45(7)106)103-75(117)59(35-47-25-27-50(108)28-26-47)96-74(116)61(37-49-40-91-54-21-14-12-19-52(49)54)97-72(114)58(34-42(2)3)92-46(8)107)78(120)95-55(22-15-30-88-82(84)85)69(111)94-57(29-33-125-9)70(112)100-63(41-105)77(119)98-60(36-48-39-90-53-20-13-11-18-51(48)53)73(115)93-56(23-16-31-89-83(86)87)71(113)101-66(43(4)5)80(122)104-32-17-24-64(104)81(123)124/h11-14,18-21,25-28,39-40,42-45,55-64,66-68,90-91,105-106,108H,10,15-17,22-24,29-38,41H2,1-9H3,(H,92,107)(H,93,115)(H,94,111)(H,95,120)(H,96,116)(H,97,114)(H,98,119)(H,99,121)(H,100,112)(H,101,113)(H,102,118)(H,103,117)(H,109,110)(H,123,124)(H4,84,85,88)(H4,86,87,89)/t44-,45+,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,66-,67-,68-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
Opioid receptor mu 1 affinity against the receptor site model site 1 (mu1) by using the curve-fitting program LIGAND |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592247
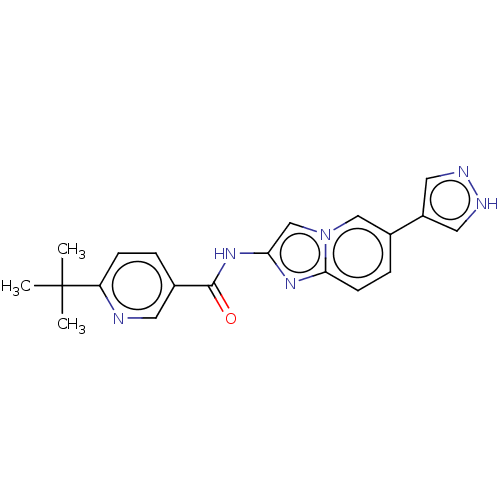
(CHEMBL5200367)Show SMILES CC(C)(C)c1ccc(cn1)C(=O)Nc1cn2cc(ccc2n1)-c1cn[nH]c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50592247

(CHEMBL5200367)Show SMILES CC(C)(C)c1ccc(cn1)C(=O)Nc1cn2cc(ccc2n1)-c1cn[nH]c1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50239363

(CHEMBL4094351)Show SMILES [H][C@]12C[C@@H](CO)N(C1)c1cc(Nc3cc4CCC(=O)Nc4c(OC\C=C\CO2)c3)n2ncc(C#N)c2n1 |r,t:26| Show InChI InChI=1S/C25H25N7O4/c26-11-16-12-27-32-22-10-21(29-25(16)32)31-13-19(9-18(31)14-33)35-5-1-2-6-36-20-8-17(28-22)7-15-3-4-23(34)30-24(15)20/h1-2,7-8,10,12,18-19,28,33H,3-6,9,13-14H2,(H,30,34)/b2-1+/t18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592235

(CHEMBL5205505) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK4
(Homo sapiens (Human)) | BDBM50592236
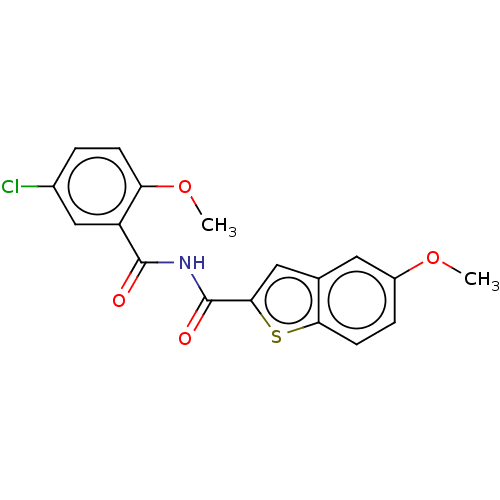
(CHEMBL5206943) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592248
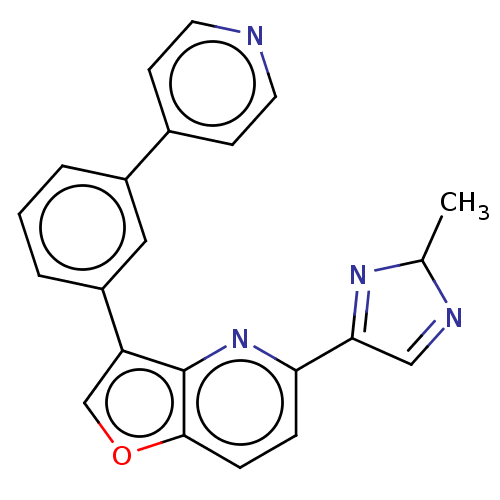
(CHEMBL5178079)Show SMILES CC1N=CC(=N1)c1ccc2occ(-c3cccc(c3)-c3ccncc3)c2n1 |c:2,4| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50602185
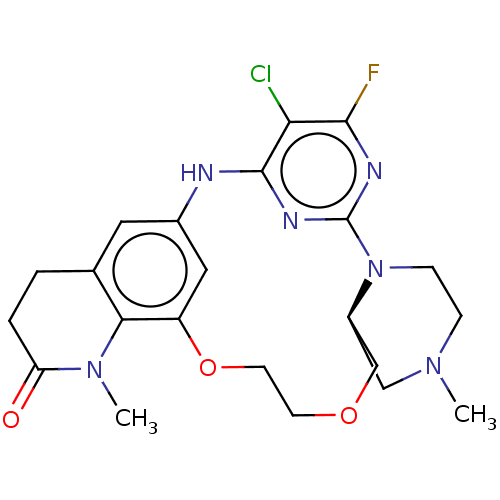
(CHEMBL5184651)Show SMILES CN1CCN2[C@H](C1)COCCOc1cc(Nc3nc2nc(F)c3Cl)cc2CCC(=O)N(C)c12 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592236

(CHEMBL5206943) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50239362
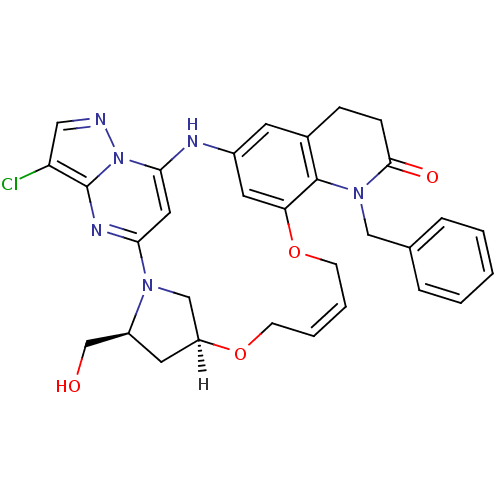
(CHEMBL4064865)Show SMILES [H][C@]12C[C@@H](CO)N(C1)c1cc(Nc3cc4CCC(=O)N(Cc5ccccc5)c4c(OC\C=C\CO2)c3)n2ncc(Cl)c2n1 |r,t:34| Show InChI InChI=1S/C31H31ClN6O4/c32-25-16-33-38-28-15-27(35-31(25)38)36-18-24(14-23(36)19-39)41-10-4-5-11-42-26-13-22(34-28)12-21-8-9-29(40)37(30(21)26)17-20-6-2-1-3-7-20/h1-7,12-13,15-16,23-24,34,39H,8-11,14,17-19H2/b5-4+/t23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50239385
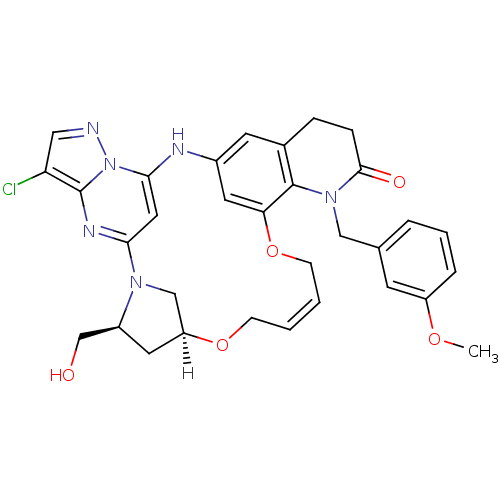
(CHEMBL4092565)Show SMILES [H][C@]12C[C@@H](CO)N(C1)c1cc(Nc3cc4CCC(=O)N(Cc5cccc(OC)c5)c4c(OC\C=C\CO2)c3)n2ncc(Cl)c2n1 |r,t:36| Show InChI InChI=1S/C32H33ClN6O5/c1-42-24-6-4-5-20(11-24)17-38-30(41)8-7-21-12-22-13-27(31(21)38)44-10-3-2-9-43-25-14-23(19-40)37(18-25)28-15-29(35-22)39-32(36-28)26(33)16-34-39/h2-6,11-13,15-16,23,25,35,40H,7-10,14,17-19H2,1H3/b3-2+/t23-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50391222
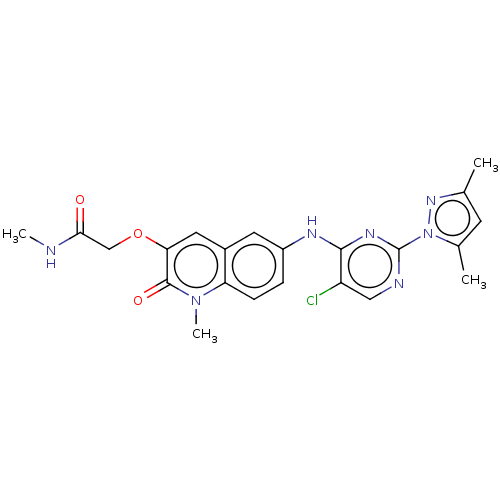
(CHEMBL5286830)Show InChI InChI=1S/C18H18N4O2/c19-17-16(10-21-18(20)22-17)24-12-14-6-8-15(9-7-14)23-11-13-4-2-1-3-5-13/h1-10H,11-12H2,(H4,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Mus musculus) | BDBM50026622

(CHEMBL408982)Show InChI InChI=1S/C13H15NO2S/c1-4-14-11-8-10(16-3)5-6-12(11)17-13(14)7-9(2)15/h5-8H,4H2,1-3H3/b13-7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK4
(Homo sapiens (Human)) | BDBM50592248

(CHEMBL5178079)Show SMILES CC1N=CC(=N1)c1ccc2occ(-c3cccc(c3)-c3ccncc3)c2n1 |c:2,4| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50239369

(CHEMBL4100303)Show SMILES [H][C@]12C[C@@H](CO)N(C1)c1cc(Nc3cc4CCC(=O)N(Cc5ccccc5C)c4c(OC\C=C\CO2)c3)n2ncc(Cl)c2n1 |r,t:35| Show InChI InChI=1S/C32H33ClN6O4/c1-20-6-2-3-7-22(20)17-38-30(41)9-8-21-12-23-13-27(31(21)38)43-11-5-4-10-42-25-14-24(19-40)37(18-25)28-15-29(35-23)39-32(36-28)26(33)16-34-39/h2-7,12-13,15-16,24-25,35,40H,8-11,14,17-19H2,1H3/b5-4+/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592249

(CHEMBL5191395) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK4
(Mus musculus) | BDBM50026622

(CHEMBL408982)Show InChI InChI=1S/C13H15NO2S/c1-4-14-11-8-10(16-3)5-6-12(11)17-13(14)7-9(2)15/h5-8H,4H2,1-3H3/b13-7- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50592246

(T3-CLK)Show SMILES CN1CCN(CC1)C(=O)C(C)(C)c1ccc(cc1)C(=O)Nc1cn2cc(ccc2n1)-c1ccncc1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50321752

(5-Chloro-2-(2-methoxyphenyl)-4H-benzo[d][1,3]oxazi...)Show InChI InChI=1S/C15H10ClNO3/c1-19-12-8-3-2-5-9(12)14-17-11-7-4-6-10(16)13(11)15(18)20-14/h2-8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 2 mins by ELISA |
Eur J Med Chem 45: 3111-5 (2010)
Article DOI: 10.1016/j.ejmech.2010.03.046
BindingDB Entry DOI: 10.7270/Q2251JCW |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592237

(CHEMBL5172542) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50592248

(CHEMBL5178079)Show SMILES CC1N=CC(=N1)c1ccc2occ(-c3cccc(c3)-c3ccncc3)c2n1 |c:2,4| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Mus musculus) | BDBM50026622

(CHEMBL408982)Show InChI InChI=1S/C13H15NO2S/c1-4-14-11-8-10(16-3)5-6-12(11)17-13(14)7-9(2)15/h5-8H,4H2,1-3H3/b13-7- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592238

(CHEMBL5191525) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50321750

(5-Chloro-2-(2-bromophenyl)-4H-benzo[d][1,3]oxazin-...)Show InChI InChI=1S/C14H7BrClNO2/c15-9-5-2-1-4-8(9)13-17-11-7-3-6-10(16)12(11)14(18)19-13/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase after 30 mins by ELISA |
Eur J Med Chem 45: 3111-5 (2010)
Article DOI: 10.1016/j.ejmech.2010.03.046
BindingDB Entry DOI: 10.7270/Q2251JCW |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50321749
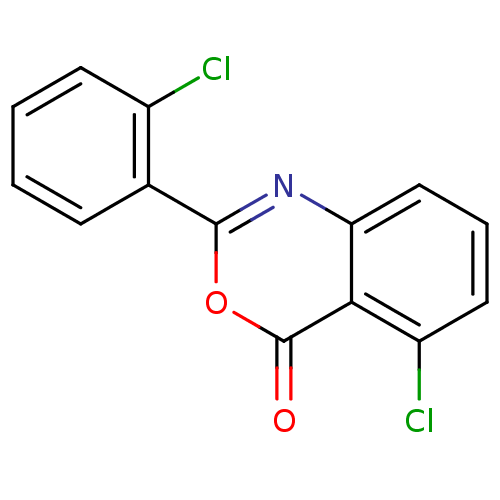
(5-Chloro-2-(2-chlorophenyl)-4H-benzo[d][1,3]oxazin...)Show InChI InChI=1S/C14H7Cl2NO2/c15-9-5-2-1-4-8(9)13-17-11-7-3-6-10(16)12(11)14(18)19-13/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 2 mins by ELISA |
Eur J Med Chem 45: 3111-5 (2010)
Article DOI: 10.1016/j.ejmech.2010.03.046
BindingDB Entry DOI: 10.7270/Q2251JCW |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50321750

(5-Chloro-2-(2-bromophenyl)-4H-benzo[d][1,3]oxazin-...)Show InChI InChI=1S/C14H7BrClNO2/c15-9-5-2-1-4-8(9)13-17-11-7-3-6-10(16)12(11)14(18)19-13/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 2 mins by ELISA |
Eur J Med Chem 45: 3111-5 (2010)
Article DOI: 10.1016/j.ejmech.2010.03.046
BindingDB Entry DOI: 10.7270/Q2251JCW |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50321749

(5-Chloro-2-(2-chlorophenyl)-4H-benzo[d][1,3]oxazin...)Show InChI InChI=1S/C14H7Cl2NO2/c15-9-5-2-1-4-8(9)13-17-11-7-3-6-10(16)12(11)14(18)19-13/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase after 30 mins by ELISA |
Eur J Med Chem 45: 3111-5 (2010)
Article DOI: 10.1016/j.ejmech.2010.03.046
BindingDB Entry DOI: 10.7270/Q2251JCW |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50321748
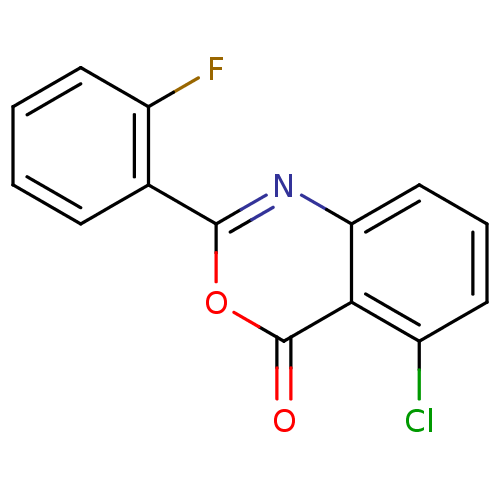
(5-Chloro-2-(2-fluorophenyl)-4H-benzo[d][1,3]oxazin...)Show InChI InChI=1S/C14H7ClFNO2/c15-9-5-3-7-11-12(9)14(18)19-13(17-11)8-4-1-2-6-10(8)16/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 2 mins by ELISA |
Eur J Med Chem 45: 3111-5 (2010)
Article DOI: 10.1016/j.ejmech.2010.03.046
BindingDB Entry DOI: 10.7270/Q2251JCW |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50230693

(CHEMBL5269254)Show SMILES CCCN(CCC)CCC1CCN(CC(=O)N2c3ccccc3NC(=O)c3ccccc23)CC1 Show InChI InChI=1S/C28H38N4O2/c1-3-16-30(17-4-2)18-13-22-14-19-31(20-15-22)21-27(33)32-25-11-7-5-9-23(25)28(34)29-24-10-6-8-12-26(24)32/h5-12,22H,3-4,13-21H2,1-2H3,(H,29,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
Opioid receptor affinity against Opioid receptor kappa 1 by using the curve-fitting program LIGAND |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592239
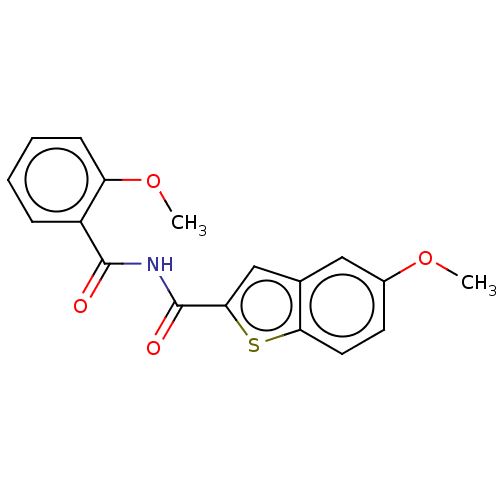
(CHEMBL5183331) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50321752

(5-Chloro-2-(2-methoxyphenyl)-4H-benzo[d][1,3]oxazi...)Show InChI InChI=1S/C15H10ClNO3/c1-19-12-8-3-2-5-9(12)14-17-11-7-4-6-10(16)13(11)15(18)20-14/h2-8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase after 30 mins by ELISA |
Eur J Med Chem 45: 3111-5 (2010)
Article DOI: 10.1016/j.ejmech.2010.03.046
BindingDB Entry DOI: 10.7270/Q2251JCW |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50391220

(CHEMBL5277976)Show InChI InChI=1S/C12H14N4/c1-8-10(11(13)16-12(14)15-8)7-9-5-3-2-4-6-9/h2-6H,7H2,1H3,(H4,13,14,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50321751
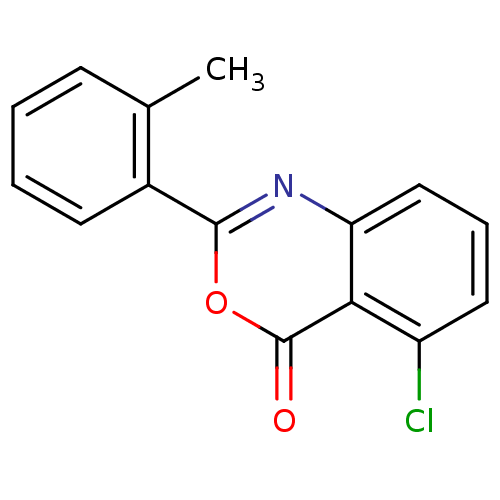
(5-Chloro-2-(2-methylphenyl)-4H-benzo[d][1,3]oxazin...)Show InChI InChI=1S/C15H10ClNO2/c1-9-5-2-3-6-10(9)14-17-12-8-4-7-11(16)13(12)15(18)19-14/h2-8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University
Curated by ChEMBL
| Assay Description
Inhibition of human leukocyte elastase after 2 mins by ELISA |
Eur J Med Chem 45: 3111-5 (2010)
Article DOI: 10.1016/j.ejmech.2010.03.046
BindingDB Entry DOI: 10.7270/Q2251JCW |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592240
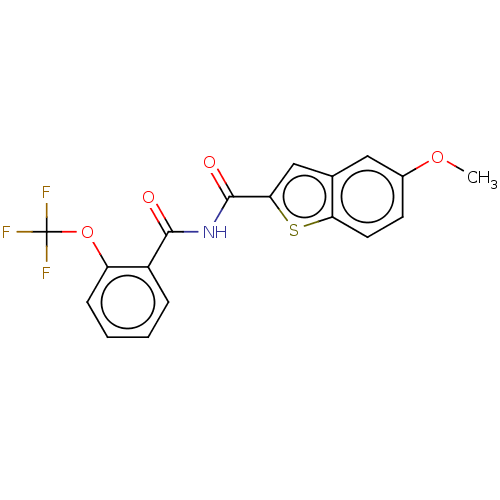
(CHEMBL5177305)Show SMILES COc1ccc2sc(cc2c1)C(=O)NC(=O)c1ccccc1OC(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50063047
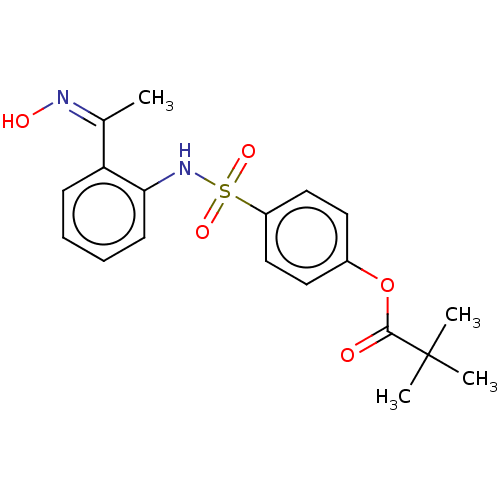
(CHEMBL3398148)Show SMILES C\C(=N\O)c1ccccc1NS(=O)(=O)c1ccc(OC(=O)C(C)(C)C)cc1 Show InChI InChI=1S/C19H22N2O5S/c1-13(20-23)16-7-5-6-8-17(16)21-27(24,25)15-11-9-14(10-12-15)26-18(22)19(2,3)4/h5-12,21,23H,1-4H3/b20-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase measured for 30 mins by spectrophotometry |
Bioorg Med Chem 23: 1123-34 (2015)
Article DOI: 10.1016/j.bmc.2014.12.056
BindingDB Entry DOI: 10.7270/Q2CJ8G5M |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50321748

(5-Chloro-2-(2-fluorophenyl)-4H-benzo[d][1,3]oxazin...)Show InChI InChI=1S/C14H7ClFNO2/c15-9-5-3-7-11-12(9)14(18)19-13(17-11)8-4-1-2-6-10(8)16/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase after 30 mins by ELISA |
Eur J Med Chem 45: 3111-5 (2010)
Article DOI: 10.1016/j.ejmech.2010.03.046
BindingDB Entry DOI: 10.7270/Q2251JCW |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50321751

(5-Chloro-2-(2-methylphenyl)-4H-benzo[d][1,3]oxazin...)Show InChI InChI=1S/C15H10ClNO2/c1-9-5-2-3-6-10(9)14-17-12-8-4-7-11(16)13(12)15(18)19-14/h2-8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase after 30 mins by ELISA |
Eur J Med Chem 45: 3111-5 (2010)
Article DOI: 10.1016/j.ejmech.2010.03.046
BindingDB Entry DOI: 10.7270/Q2251JCW |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50391221

(CHEMBL5273385)Show InChI InChI=1S/C13H16N4/c1-9-11(12(14)17-13(15)16-9)8-7-10-5-3-2-4-6-10/h2-6H,7-8H2,1H3,(H4,14,15,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-2 adrenergic receptor was measured by the inhibition of isoproterenol-induced relaxation of PGF2-alpha cont... |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592241
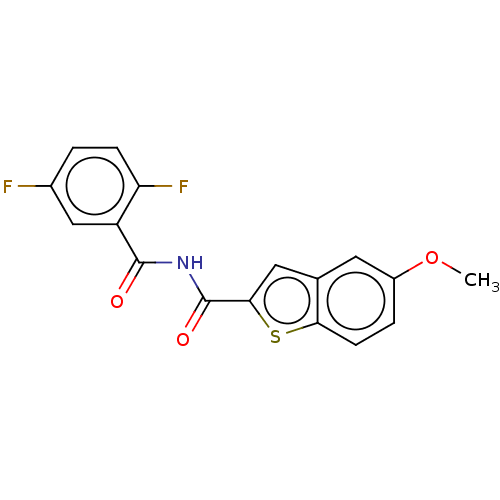
(CHEMBL5206862) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50084637

(2,2-Dimethyl-propionic acid 4-[2-(carboxymethyl-ca...)Show SMILES CC(C)(C)C(=O)Oc1ccc(cc1)S(=O)(=O)Nc1ccccc1C(=O)NCC(O)=O Show InChI InChI=1S/C20H22N2O7S/c1-20(2,3)19(26)29-13-8-10-14(11-9-13)30(27,28)22-16-7-5-4-6-15(16)18(25)21-12-17(23)24/h4-11,22H,12H2,1-3H3,(H,21,25)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase measured for 30 mins by spectrophotometry |
Bioorg Med Chem 23: 1123-34 (2015)
Article DOI: 10.1016/j.bmc.2014.12.056
BindingDB Entry DOI: 10.7270/Q2CJ8G5M |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592242

(CHEMBL5174345) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592243
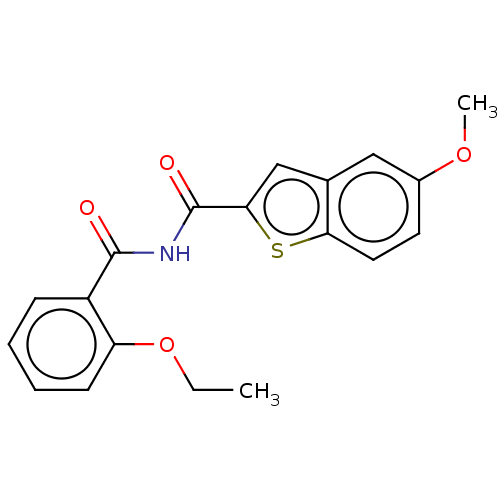
(CHEMBL5181998) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50239369

(CHEMBL4100303)Show SMILES [H][C@]12C[C@@H](CO)N(C1)c1cc(Nc3cc4CCC(=O)N(Cc5ccccc5C)c4c(OC\C=C\CO2)c3)n2ncc(Cl)c2n1 |r,t:35| Show InChI InChI=1S/C32H33ClN6O4/c1-20-6-2-3-7-22(20)17-38-30(41)9-8-21-12-23-13-27(31(21)38)43-11-5-4-10-42-25-14-24(19-40)37(18-25)28-15-29(35-23)39-32(36-28)26(33)16-34-39/h2-7,12-13,15-16,24-25,35,40H,8-11,14,17-19H2,1H3/b5-4+/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592244
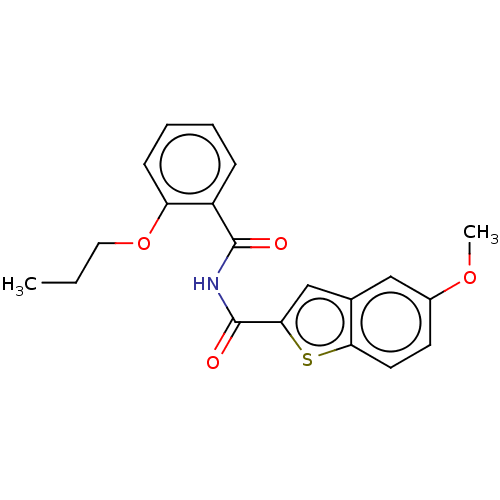
(CHEMBL5194284) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50592245
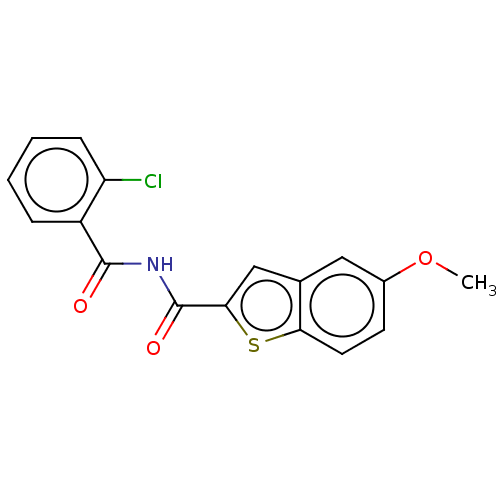
(CHEMBL5200302) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50230700
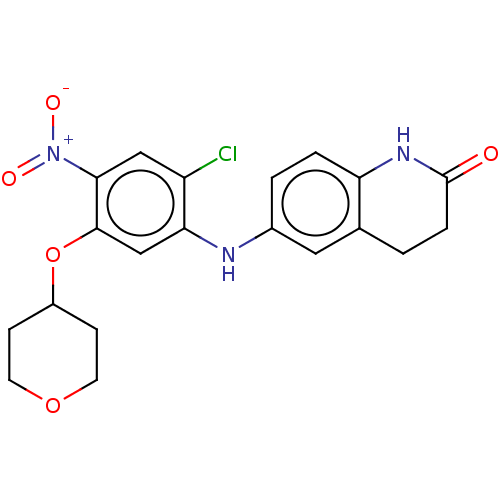
(CHEMBL4075278)Show SMILES CCCCN(CCCC)CCC1CCN(CC(=O)N2c3ccccc3NC(=O)c3ccccc23)CC1 Show InChI InChI=1S/C30H42N4O2/c1-3-5-18-32(19-6-4-2)20-15-24-16-21-33(22-17-24)23-29(35)34-27-13-9-7-11-25(27)30(36)31-26-12-8-10-14-28(26)34/h7-14,24H,3-6,15-23H2,1-2H3,(H,31,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50239385

(CHEMBL4092565)Show SMILES [H][C@]12C[C@@H](CO)N(C1)c1cc(Nc3cc4CCC(=O)N(Cc5cccc(OC)c5)c4c(OC\C=C\CO2)c3)n2ncc(Cl)c2n1 |r,t:36| Show InChI InChI=1S/C32H33ClN6O5/c1-42-24-6-4-5-20(11-24)17-38-30(41)8-7-21-12-22-13-27(31(21)38)44-10-3-2-9-43-25-14-23(19-40)37(18-25)28-15-29(35-22)39-32(36-28)26(33)16-34-39/h2-6,11-13,15-16,23,25,35,40H,7-10,14,17-19H2,1H3/b3-2+/t23-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50063052

(CHEMBL3398143)Show SMILES CC(=O)c1ccccc1NS(=O)(=O)c1ccc(OC(=O)C(C)(C)C)cc1 Show InChI InChI=1S/C19H21NO5S/c1-13(21)16-7-5-6-8-17(16)20-26(23,24)15-11-9-14(10-12-15)25-18(22)19(2,3)4/h5-12,20H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University
Curated by ChEMBL
| Assay Description
Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry |
Bioorg Med Chem 23: 1123-34 (2015)
Article DOI: 10.1016/j.bmc.2014.12.056
BindingDB Entry DOI: 10.7270/Q2CJ8G5M |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50602186

(CHEMBL5207356)Show SMILES CN1C(=O)CCc2cc3Nc4nc(ncc4Cl)N4CCN(C[C@@H]4COCCOc(c3)c12)C(=O)CCC(=O)NCCCOCCOCCOc1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cia SA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against beta-1 adrenergic receptor measured by inhibition of positive chronotropic effect of isoproterenolin in isolated... |
J Med Chem 39: 2197-206 (1996)
Article DOI: 10.1021/jm9508853 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data