

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM222462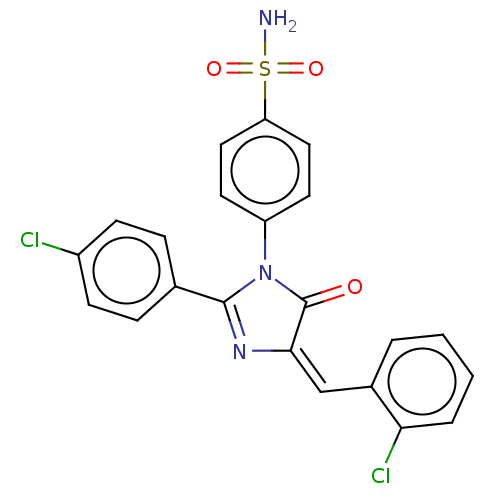 ((E)-4-[4-(2-Chlorobenzylidene)-2-(4-chlorophenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 by EIA | Eur J Med Chem 173: 117-153 (2019) Article DOI: 10.1016/j.ejmech.2019.03.063 BindingDB Entry DOI: 10.7270/Q2QJ7MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50502118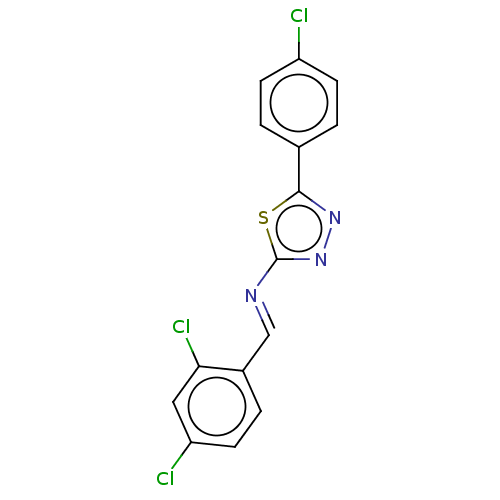 (CHEMBL4476702) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using p-nitrophenyl-a-d-glucopyranoside as substrate preincubated for 5 mins followed by substrate a... | Eur J Med Chem 173: 117-153 (2019) Article DOI: 10.1016/j.ejmech.2019.03.063 BindingDB Entry DOI: 10.7270/Q2QJ7MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM222462 ((E)-4-[4-(2-Chlorobenzylidene)-2-(4-chlorophenyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of ovine COX1 by EIA | Eur J Med Chem 173: 117-153 (2019) Article DOI: 10.1016/j.ejmech.2019.03.063 BindingDB Entry DOI: 10.7270/Q2QJ7MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50512315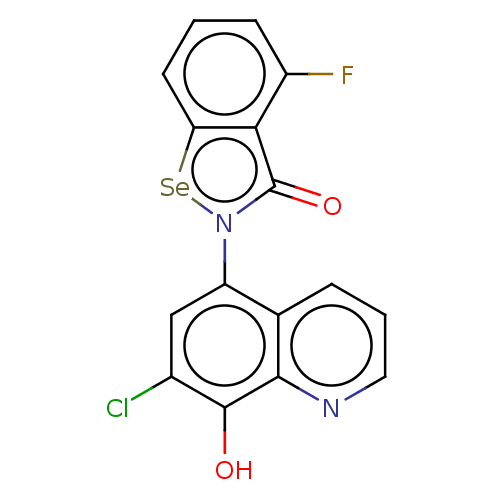 (CHEMBL4438040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of amyloid beta (1 to 42) (unknown origin) aggregation | Eur J Med Chem 173: 117-153 (2019) Article DOI: 10.1016/j.ejmech.2019.03.063 BindingDB Entry DOI: 10.7270/Q2QJ7MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Homo sapiens (Human)) | BDBM178133 (3-(6-Chloro-1H-imidazo[4,5-b]pyridin-2-yl)benzene-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of beta-glucuronidase (unknown origin) using p-nitrophenyl-beta-D-glucuronide as substrate pre-incubated for 30 mins followed by substrate... | Eur J Med Chem 173: 117-153 (2019) Article DOI: 10.1016/j.ejmech.2019.03.063 BindingDB Entry DOI: 10.7270/Q2QJ7MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||