

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50465712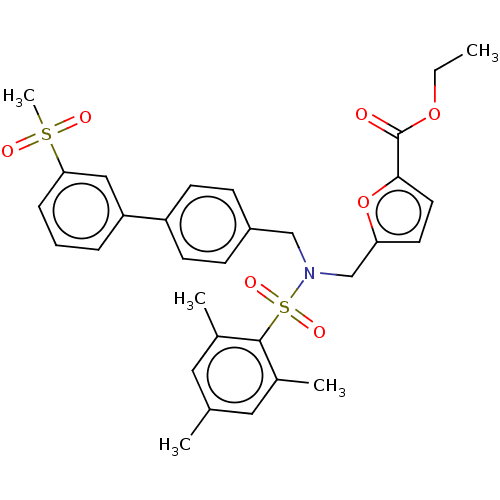 (CHEMBL4284414) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Inverse agonist activity at LXRbeta (unknown origin) expressed in HEK293 cells 24 hrs by Dual-Glo luciferase assay | J Med Chem 61: 10935-10956 (2018) Article DOI: 10.1021/acs.jmedchem.8b00045 BindingDB Entry DOI: 10.7270/Q2SX6GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50465712 (CHEMBL4284414) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Inverse agonist activity at LXRalpha (unknown origin) expressed in HEK293 cells 24 hrs by Dual-Glo luciferase assay | J Med Chem 61: 10935-10956 (2018) Article DOI: 10.1021/acs.jmedchem.8b00045 BindingDB Entry DOI: 10.7270/Q2SX6GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50396601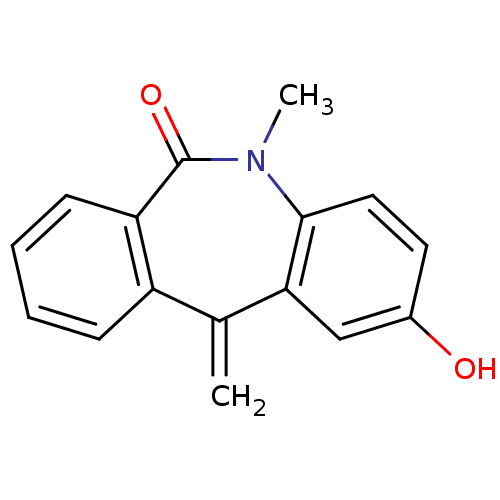 (CHEMBL2171902) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Antagonist activity at human LXRalpha expressed in HEK293T cells by luciferase reporter gene assay | J Med Chem 61: 10935-10956 (2018) Article DOI: 10.1021/acs.jmedchem.8b00045 BindingDB Entry DOI: 10.7270/Q2SX6GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50465711 (CHEMBL1316881) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Agonist activity at human LXRalpha-LBD (182 to 447 amino acids) expressed in HEK293T cells by luciferase reporter gene assay | J Med Chem 61: 10935-10956 (2018) Article DOI: 10.1021/acs.jmedchem.8b00045 BindingDB Entry DOI: 10.7270/Q2SX6GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50465713 (CHEMBL4277633 | US20230340011, Example 94.) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Agonist activity at human LXRalpha expressed in HEK293 cells after 24 hrs by One-Glo luciferase reporter gene assay | J Med Chem 61: 10935-10956 (2018) Article DOI: 10.1021/acs.jmedchem.8b00045 BindingDB Entry DOI: 10.7270/Q2SX6GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50465711 (CHEMBL1316881) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Agonist activity at human LXRbeta-LBD (196 to 461 amino acids) expressed in HEK293T cells by luciferase reporter gene assay | J Med Chem 61: 10935-10956 (2018) Article DOI: 10.1021/acs.jmedchem.8b00045 BindingDB Entry DOI: 10.7270/Q2SX6GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034786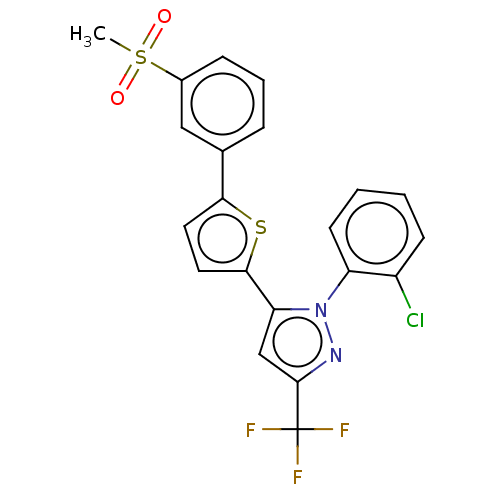 (CHEMBL3360964) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 108 | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Agonist activity at human full length LXRbeta expressed in African green monkey CV-1 cells co-expressing human pCMX/RXRalpha after 20 hrs by lucifera... | J Med Chem 61: 10935-10956 (2018) Article DOI: 10.1021/acs.jmedchem.8b00045 BindingDB Entry DOI: 10.7270/Q2SX6GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50465710 (CHEMBL4289727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 720 | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Agonist activity at GAL4-tagged human LXRbeta LBD expressed in HEK293T cells after 12 hrs by luciferase reporter gene assay | J Med Chem 61: 10935-10956 (2018) Article DOI: 10.1021/acs.jmedchem.8b00045 BindingDB Entry DOI: 10.7270/Q2SX6GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20186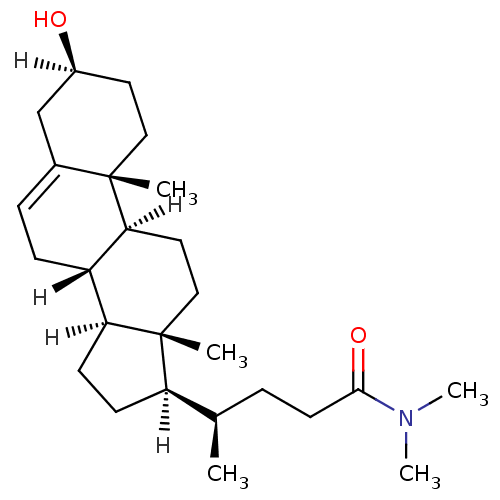 ((4R)-4-[(1S,2R,5S,10S,11S,14R,15R)-5-hydroxy-2,15-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 422 | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Agonist activity at human LXRalpha expressed in HEK293 cells after 24 hrs by One-Glo luciferase reporter gene assay | J Med Chem 61: 10935-10956 (2018) Article DOI: 10.1021/acs.jmedchem.8b00045 BindingDB Entry DOI: 10.7270/Q2SX6GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||