 Found 113 hits with Last Name = 'chico' and Initial = 'lw'
Found 113 hits with Last Name = 'chico' and Initial = 'lw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50537592

(CHEMBL4632881)Show SMILES C1CN(CCN1c1cc(-c2ccncc2)c(nn1)-c1ccccc1)c1ncccn1 Show InChI InChI=1S/C23H21N7/c1-2-5-19(6-3-1)22-20(18-7-11-24-12-8-18)17-21(27-28-22)29-13-15-30(16-14-29)23-25-9-4-10-26-23/h1-12,17H,13-16H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y181C reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537600

(CHEMBL4129018 | US11149020, Compound 27 (MW-150))Show SMILES CN1CCN(CC1)c1cc(-c2ccncc2)c(nn1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C24H23N5/c1-28-12-14-29(15-13-28)23-17-22(19-8-10-25-11-9-19)24(27-26-23)21-7-6-18-4-2-3-5-20(18)16-21/h2-11,16-17H,12-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y188L reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537599
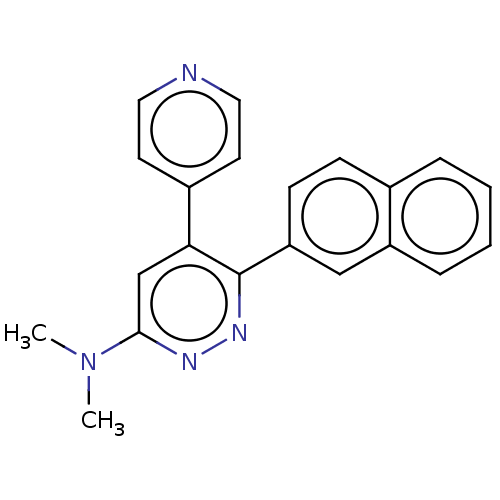
(CHEMBL4648060 | US11149020, Compound 2 (MW-108))Show InChI InChI=1S/C21H18N4/c1-25(2)20-14-19(16-9-11-22-12-10-16)21(24-23-20)18-8-7-15-5-3-4-6-17(15)13-18/h3-14H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537598

(CHEMBL4646628 | US11149020, Compound 1 (MW-181))Show InChI InChI=1S/C21H18N4/c1-25(2)20-14-19(16-10-12-22-13-11-16)21(24-23-20)18-9-5-7-15-6-3-4-8-17(15)18/h3-14H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Mutant Reverse transcriptase P236L |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50537598

(CHEMBL4646628 | US11149020, Compound 1 (MW-181))Show InChI InChI=1S/C21H18N4/c1-25(2)20-14-19(16-10-12-22-13-11-16)21(24-23-20)18-9-5-7-15-6-3-4-8-17(15)18/h3-14H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y188L reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537592

(CHEMBL4632881)Show SMILES C1CN(CCN1c1cc(-c2ccncc2)c(nn1)-c1ccccc1)c1ncccn1 Show InChI InChI=1S/C23H21N7/c1-2-5-19(6-3-1)22-20(18-7-11-24-12-8-18)17-21(27-28-22)29-13-15-30(16-14-29)23-25-9-4-10-26-23/h1-12,17H,13-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Mutant Reverse transcriptase K103N |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50537594
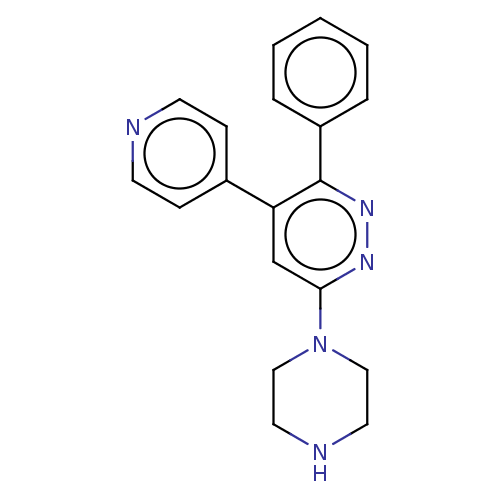
(CHEMBL4639028)Show InChI InChI=1S/C19H19N5/c1-2-4-16(5-3-1)19-17(15-6-8-20-9-7-15)14-18(22-23-19)24-12-10-21-11-13-24/h1-9,14,21H,10-13H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y181C reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50537593

(CHEMBL4639555 | US11149020, Compound 3 (MW-066))Show InChI InChI=1S/C20H21N5/c1-24-11-13-25(14-12-24)19-15-18(16-7-9-21-10-8-16)20(23-22-19)17-5-3-2-4-6-17/h2-10,15H,11-14H2,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 wild-type reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537594

(CHEMBL4639028)Show InChI InChI=1S/C19H19N5/c1-2-4-16(5-3-1)19-17(15-6-8-20-9-7-15)14-18(22-23-19)24-12-10-21-11-13-24/h1-9,14,21H,10-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537606

(CHEMBL4647072 | US11149020, Compound 34 (MW-154))Show SMILES C1CN(CCN1)c1cc(-c2ccncc2)c(nn1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C23H21N5/c1-2-4-19-15-20(6-5-17(19)3-1)23-21(18-7-9-24-10-8-18)16-22(26-27-23)28-13-11-25-12-14-28/h1-10,15-16,25H,11-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 wild-type reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537598

(CHEMBL4646628 | US11149020, Compound 1 (MW-181))Show InChI InChI=1S/C21H18N4/c1-25(2)20-14-19(16-10-12-22-13-11-16)21(24-23-20)18-9-5-7-15-6-3-4-8-17(15)18/h3-14H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 wild-type reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537608
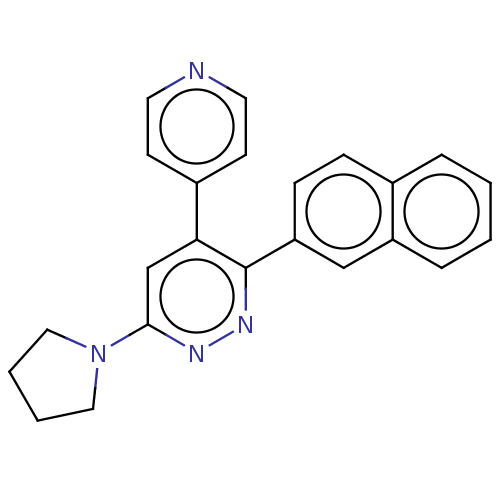
(CHEMBL4639995 | US11149020, Compound 32 (MW-148))Show SMILES C1CCN(C1)c1cc(-c2ccncc2)c(nn1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C23H20N4/c1-2-6-19-15-20(8-7-17(19)5-1)23-21(18-9-11-24-12-10-18)16-22(25-26-23)27-13-3-4-14-27/h1-2,5-12,15-16H,3-4,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y181C reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537599

(CHEMBL4648060 | US11149020, Compound 2 (MW-108))Show InChI InChI=1S/C21H18N4/c1-25(2)20-14-19(16-9-11-22-12-10-16)21(24-23-20)18-8-7-15-5-3-4-6-17(15)13-18/h3-14H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y181C reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50537592

(CHEMBL4632881)Show SMILES C1CN(CCN1c1cc(-c2ccncc2)c(nn1)-c1ccccc1)c1ncccn1 Show InChI InChI=1S/C23H21N7/c1-2-5-19(6-3-1)22-20(18-7-11-24-12-8-18)17-21(27-28-22)29-13-15-30(16-14-29)23-25-9-4-10-26-23/h1-12,17H,13-16H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y181C reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537600

(CHEMBL4129018 | US11149020, Compound 27 (MW-150))Show SMILES CN1CCN(CC1)c1cc(-c2ccncc2)c(nn1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C24H23N5/c1-28-12-14-29(15-13-28)23-17-22(19-8-10-25-11-9-19)24(27-26-23)21-7-6-18-4-2-3-5-20(18)16-21/h2-11,16-17H,12-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y181C reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50537596
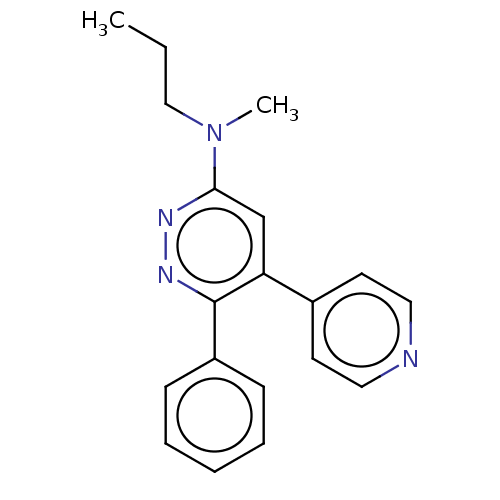
(CHEMBL4648747 | US11149020, Compound 5 (MW-207))Show InChI InChI=1S/C19H20N4/c1-3-13-23(2)18-14-17(15-9-11-20-12-10-15)19(22-21-18)16-7-5-4-6-8-16/h4-12,14H,3,13H2,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y188L reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537607
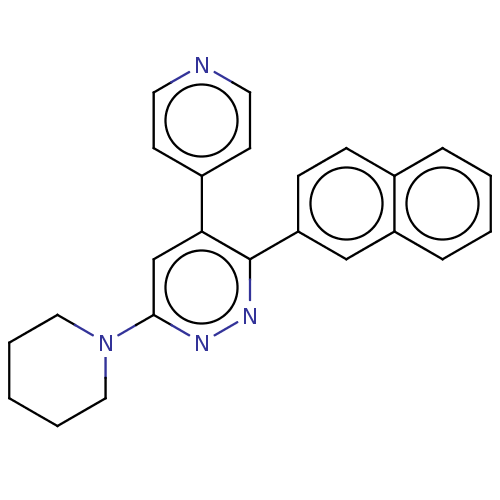
(CHEMBL4635988 | US11149020, Compound 61 (MW-086))Show SMILES C1CCN(CC1)c1cc(-c2ccncc2)c(nn1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C24H22N4/c1-4-14-28(15-5-1)23-17-22(19-10-12-25-13-11-19)24(27-26-23)21-9-8-18-6-2-3-7-20(18)16-21/h2-3,6-13,16-17H,1,4-5,14-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Mutant Reverse transcriptase K103N |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537609
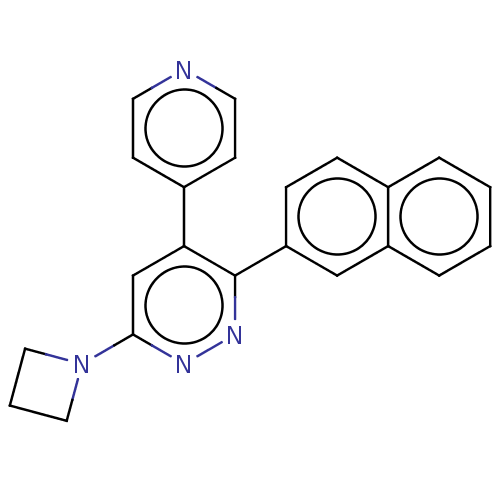
(CHEMBL4643246 | US11149020, Compound 31 (MW-146))Show SMILES C1CN(C1)c1cc(-c2ccncc2)c(nn1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C22H18N4/c1-2-5-18-14-19(7-6-16(18)4-1)22-20(17-8-10-23-11-9-17)15-21(24-25-22)26-12-3-13-26/h1-2,4-11,14-15H,3,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y181C reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537595
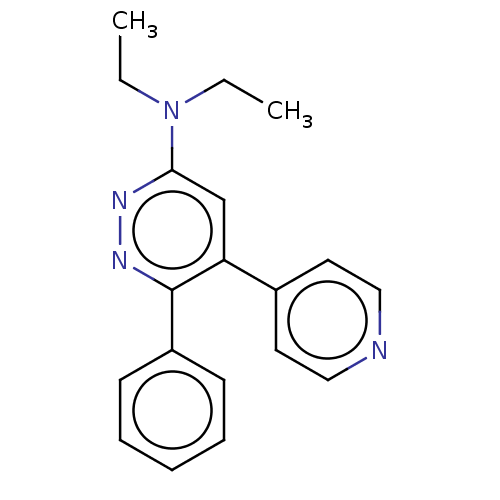
(CHEMBL4642101 | US11149020, Compound 4 (MW-177))Show InChI InChI=1S/C19H20N4/c1-3-23(4-2)18-14-17(15-10-12-20-13-11-15)19(22-21-18)16-8-6-5-7-9-16/h5-14H,3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Mutant Reverse transcriptase V106A |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50537605
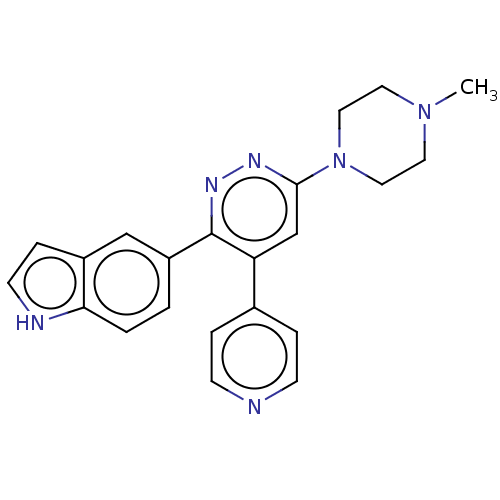
(CHEMBL4635980 | US11149020, Compound 28 (MW-118))Show SMILES CN1CCN(CC1)c1cc(-c2ccncc2)c(nn1)-c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C22H22N6/c1-27-10-12-28(13-11-27)21-15-19(16-4-7-23-8-5-16)22(26-25-21)18-2-3-20-17(14-18)6-9-24-20/h2-9,14-15,24H,10-13H2,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50537595

(CHEMBL4642101 | US11149020, Compound 4 (MW-177))Show InChI InChI=1S/C19H20N4/c1-3-23(4-2)18-14-17(15-10-12-20-13-11-15)19(22-21-18)16-8-6-5-7-9-16/h5-14H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 wild-type reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537596

(CHEMBL4648747 | US11149020, Compound 5 (MW-207))Show InChI InChI=1S/C19H20N4/c1-3-13-23(2)18-14-17(15-9-11-20-12-10-15)19(22-21-18)16-7-5-4-6-8-16/h4-12,14H,3,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y188L reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50537598

(CHEMBL4646628 | US11149020, Compound 1 (MW-181))Show InChI InChI=1S/C21H18N4/c1-25(2)20-14-19(16-10-12-22-13-11-16)21(24-23-20)18-9-5-7-15-6-3-4-8-17(15)18/h3-14H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y188L reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537597

(CHEMBL4645737 | US11149020, Compound 6 (MW-105))Show InChI InChI=1S/C17H16N4/c1-21(2)16-12-15(13-8-10-18-11-9-13)17(20-19-16)14-6-4-3-5-7-14/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 wild-type reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50537606

(CHEMBL4647072 | US11149020, Compound 34 (MW-154))Show SMILES C1CN(CCN1)c1cc(-c2ccncc2)c(nn1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C23H21N5/c1-2-4-19-15-20(6-5-17(19)3-1)23-21(18-7-9-24-10-8-18)16-22(26-27-23)28-13-11-25-12-14-28/h1-10,15-16,25H,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 wild-type reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537605

(CHEMBL4635980 | US11149020, Compound 28 (MW-118))Show SMILES CN1CCN(CC1)c1cc(-c2ccncc2)c(nn1)-c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C22H22N6/c1-27-10-12-28(13-11-27)21-15-19(16-4-7-23-8-5-16)22(26-25-21)18-2-3-20-17(14-18)6-9-24-20/h2-9,14-15,24H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537592

(CHEMBL4632881)Show SMILES C1CN(CCN1c1cc(-c2ccncc2)c(nn1)-c1ccccc1)c1ncccn1 Show InChI InChI=1S/C23H21N7/c1-2-5-19(6-3-1)22-20(18-7-11-24-12-8-18)17-21(27-28-22)29-13-15-30(16-14-29)23-25-9-4-10-26-23/h1-12,17H,13-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y181C reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537593

(CHEMBL4639555 | US11149020, Compound 3 (MW-066))Show InChI InChI=1S/C20H21N5/c1-24-11-13-25(14-12-24)19-15-18(16-7-9-21-10-8-16)20(23-22-19)17-5-3-2-4-6-17/h2-10,15H,11-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Mutant Reverse transcriptase G190A |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537603
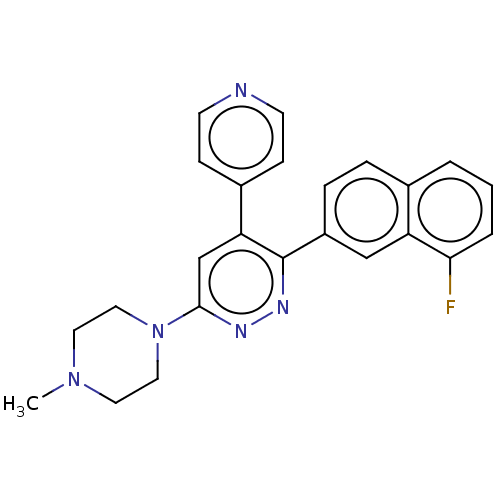
(CHEMBL4642456 | US11149020, Compound 52 (MW-032))Show SMILES CN1CCN(CC1)c1cc(-c2ccncc2)c(nn1)-c1ccc2cccc(F)c2c1 Show InChI InChI=1S/C24H22FN5/c1-29-11-13-30(14-12-29)23-16-21(18-7-9-26-10-8-18)24(28-27-23)19-6-5-17-3-2-4-22(25)20(17)15-19/h2-10,15-16H,11-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y181C reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50537597

(CHEMBL4645737 | US11149020, Compound 6 (MW-105))Show InChI InChI=1S/C17H16N4/c1-21(2)16-12-15(13-8-10-18-11-9-13)17(20-19-16)14-6-4-3-5-7-14/h3-12H,1-2H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y188L reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537602
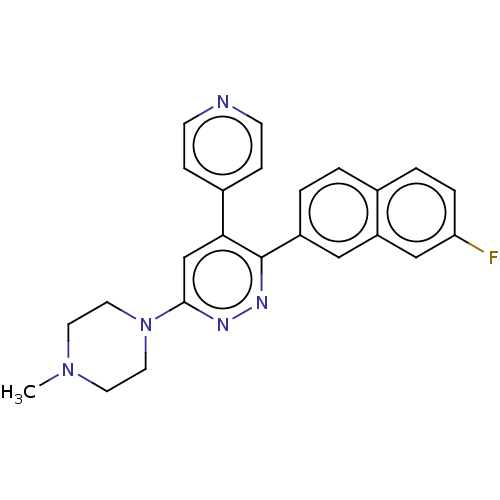
(CHEMBL4640881 | US11149020, Compound 50 (MW-017))Show SMILES CN1CCN(CC1)c1cc(-c2ccncc2)c(nn1)-c1ccc2ccc(F)cc2c1 Show InChI InChI=1S/C24H22FN5/c1-29-10-12-30(13-11-29)23-16-22(18-6-8-26-9-7-18)24(28-27-23)19-3-2-17-4-5-21(25)15-20(17)14-19/h2-9,14-16H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 wild-type reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50537601
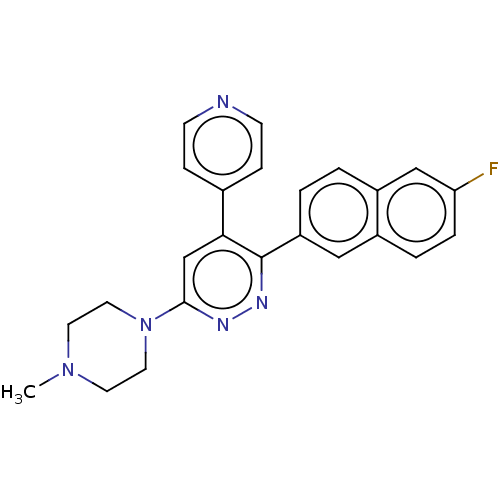
(CHEMBL4643993 | US11149020, Compound 49 (MW-203))Show SMILES CN1CCN(CC1)c1cc(-c2ccncc2)c(nn1)-c1ccc2cc(F)ccc2c1 Show InChI InChI=1S/C24H22FN5/c1-29-10-12-30(13-11-29)23-16-22(17-6-8-26-9-7-17)24(28-27-23)20-3-2-19-15-21(25)5-4-18(19)14-20/h2-9,14-16H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Mutant Reverse transcriptase V106A |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM15244
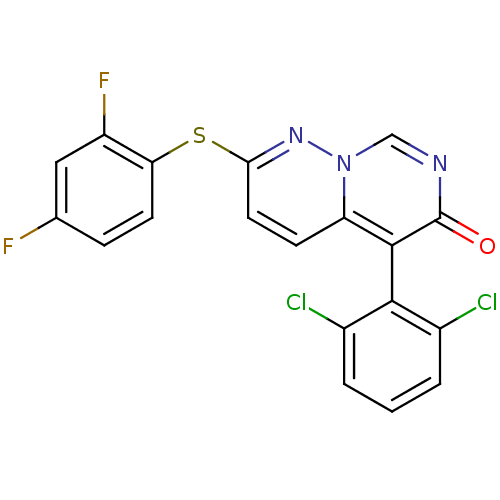
(5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfa...)Show SMILES Fc1ccc(Sc2ccc3c(-c4c(Cl)cccc4Cl)c(=O)ncn3n2)c(F)c1 |(-1.69,6.87,;-1.69,5.33,;-.36,4.56,;-.36,3.02,;-1.69,2.25,;-1.69,.71,;-3.03,-.06,;-3.03,-1.6,;-4.36,-2.37,;-5.75,-1.54,;-7.08,-2.31,;-7.08,-3.85,;-5.75,-4.62,;-4.42,-3.85,;-5.75,-6.16,;-7.08,-6.93,;-8.42,-6.16,;-8.42,-4.62,;-9.75,-3.85,;-8.42,-1.54,;-9.75,-2.31,;-8.42,,;-7.08,.77,;-5.75,,;-4.36,.71,;-3.03,3.02,;-4.36,2.25,;-3.03,4.56,)| Show InChI InChI=1S/C19H9Cl2F2N3OS/c20-11-2-1-3-12(21)17(11)18-14-5-7-16(25-26(14)9-24-19(18)27)28-15-6-4-10(22)8-13(15)23/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Y188L reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 Mutant Reverse transcriptase P236L |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50045333
(CHEBI:90705 | SB-203580)Show SMILES C[S+]([O-])c1ccc(cc1)-c1nc(c([nH]1)-c1ccncc1)-c1ccc(F)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 wild-type reverse transcriptase. |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50045333

(CHEBI:90705 | SB-203580)Show SMILES C[S+]([O-])c1ccc(cc1)-c1nc(c([nH]1)-c1ccncc1)-c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM15244

(5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfa...)Show SMILES Fc1ccc(Sc2ccc3c(-c4c(Cl)cccc4Cl)c(=O)ncn3n2)c(F)c1 |(-1.69,6.87,;-1.69,5.33,;-.36,4.56,;-.36,3.02,;-1.69,2.25,;-1.69,.71,;-3.03,-.06,;-3.03,-1.6,;-4.36,-2.37,;-5.75,-1.54,;-7.08,-2.31,;-7.08,-3.85,;-5.75,-4.62,;-4.42,-3.85,;-5.75,-6.16,;-7.08,-6.93,;-8.42,-6.16,;-8.42,-4.62,;-9.75,-3.85,;-8.42,-1.54,;-9.75,-2.31,;-8.42,,;-7.08,.77,;-5.75,,;-4.36,.71,;-3.03,3.02,;-4.36,2.25,;-3.03,4.56,)| Show InChI InChI=1S/C19H9Cl2F2N3OS/c20-11-2-1-3-12(21)17(11)18-14-5-7-16(25-26(14)9-24-19(18)27)28-15-6-4-10(22)8-13(15)23/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM15244

(5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfa...)Show SMILES Fc1ccc(Sc2ccc3c(-c4c(Cl)cccc4Cl)c(=O)ncn3n2)c(F)c1 |(-1.69,6.87,;-1.69,5.33,;-.36,4.56,;-.36,3.02,;-1.69,2.25,;-1.69,.71,;-3.03,-.06,;-3.03,-1.6,;-4.36,-2.37,;-5.75,-1.54,;-7.08,-2.31,;-7.08,-3.85,;-5.75,-4.62,;-4.42,-3.85,;-5.75,-6.16,;-7.08,-6.93,;-8.42,-6.16,;-8.42,-4.62,;-9.75,-3.85,;-8.42,-1.54,;-9.75,-2.31,;-8.42,,;-7.08,.77,;-5.75,,;-4.36,.71,;-3.03,3.02,;-4.36,2.25,;-3.03,4.56,)| Show InChI InChI=1S/C19H9Cl2F2N3OS/c20-11-2-1-3-12(21)17(11)18-14-5-7-16(25-26(14)9-24-19(18)27)28-15-6-4-10(22)8-13(15)23/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM15244

(5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfa...)Show SMILES Fc1ccc(Sc2ccc3c(-c4c(Cl)cccc4Cl)c(=O)ncn3n2)c(F)c1 |(-1.69,6.87,;-1.69,5.33,;-.36,4.56,;-.36,3.02,;-1.69,2.25,;-1.69,.71,;-3.03,-.06,;-3.03,-1.6,;-4.36,-2.37,;-5.75,-1.54,;-7.08,-2.31,;-7.08,-3.85,;-5.75,-4.62,;-4.42,-3.85,;-5.75,-6.16,;-7.08,-6.93,;-8.42,-6.16,;-8.42,-4.62,;-9.75,-3.85,;-8.42,-1.54,;-9.75,-2.31,;-8.42,,;-7.08,.77,;-5.75,,;-4.36,.71,;-3.03,3.02,;-4.36,2.25,;-3.03,4.56,)| Show InChI InChI=1S/C19H9Cl2F2N3OS/c20-11-2-1-3-12(21)17(11)18-14-5-7-16(25-26(14)9-24-19(18)27)28-15-6-4-10(22)8-13(15)23/h1-9H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM15244

(5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfa...)Show SMILES Fc1ccc(Sc2ccc3c(-c4c(Cl)cccc4Cl)c(=O)ncn3n2)c(F)c1 |(-1.69,6.87,;-1.69,5.33,;-.36,4.56,;-.36,3.02,;-1.69,2.25,;-1.69,.71,;-3.03,-.06,;-3.03,-1.6,;-4.36,-2.37,;-5.75,-1.54,;-7.08,-2.31,;-7.08,-3.85,;-5.75,-4.62,;-4.42,-3.85,;-5.75,-6.16,;-7.08,-6.93,;-8.42,-6.16,;-8.42,-4.62,;-9.75,-3.85,;-8.42,-1.54,;-9.75,-2.31,;-8.42,,;-7.08,.77,;-5.75,,;-4.36,.71,;-3.03,3.02,;-4.36,2.25,;-3.03,4.56,)| Show InChI InChI=1S/C19H9Cl2F2N3OS/c20-11-2-1-3-12(21)17(11)18-14-5-7-16(25-26(14)9-24-19(18)27)28-15-6-4-10(22)8-13(15)23/h1-9H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 8
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 7
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 8
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 12
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data