 Found 19 hits with Last Name = '?witalska' and Initial = 'm'
Found 19 hits with Last Name = '?witalska' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50604012

(CHEMBL5199004)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cn(nn1)-c1cc(F)cc(F)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM13058
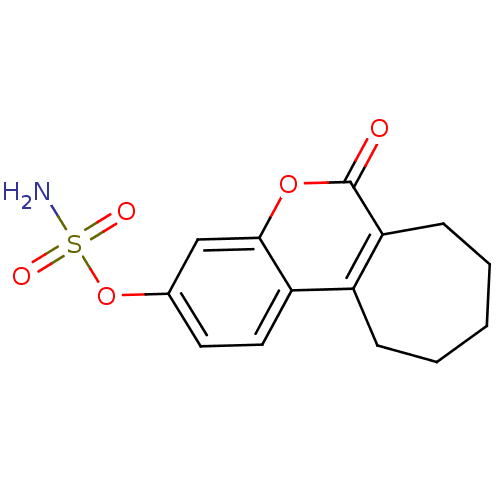
(6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...)Show InChI InChI=1S/C14H15NO5S/c15-21(17,18)20-9-6-7-11-10-4-2-1-3-5-12(10)14(16)19-13(11)8-9/h6-8H,1-5H2,(H2,15,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50604011
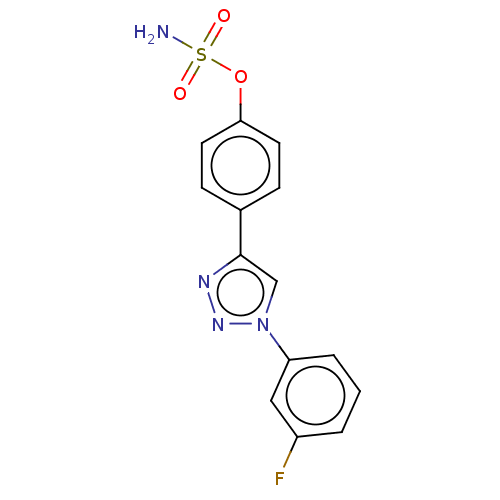
(CHEMBL5205557)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cn(nn1)-c1cccc(F)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50604009
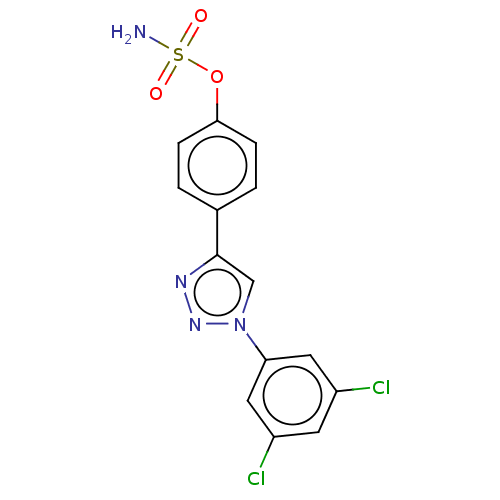
(CHEMBL5170413)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cn(nn1)-c1cc(Cl)cc(Cl)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50604008
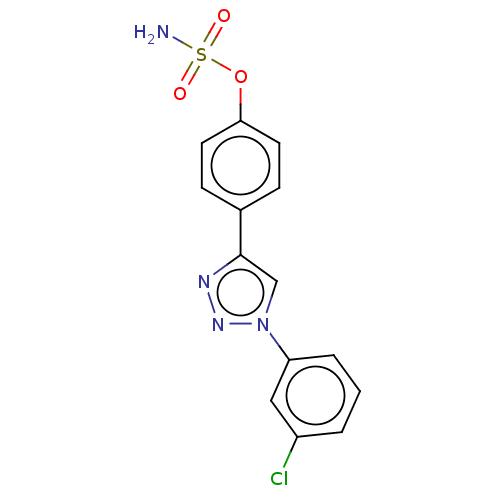
(CHEMBL5179747)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cn(nn1)-c1cccc(Cl)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50604010
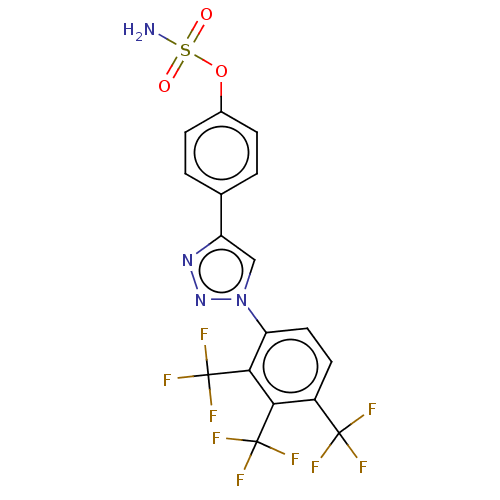
(CHEMBL5169672)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cn(nn1)-c1ccc(c(c1C(F)(F)F)C(F)(F)F)C(F)(F)F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50604012

(CHEMBL5199004)Show SMILES NS(=O)(=O)Oc1ccc(cc1)-c1cn(nn1)-c1cc(F)cc(F)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50051829
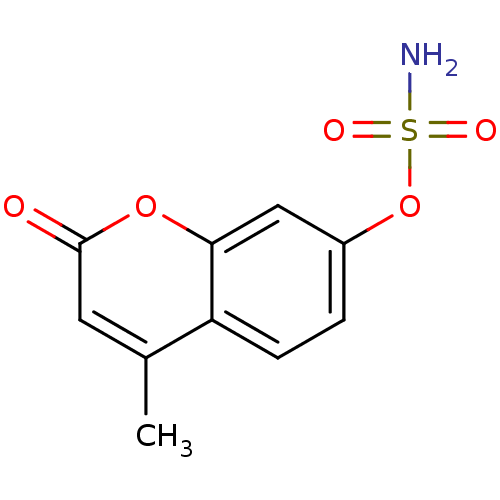
(4-methyl-2-oxo-2H-chromen-7-yl sulfamate | CHEMBL1...)Show InChI InChI=1S/C10H9NO5S/c1-6-4-10(12)15-9-5-7(2-3-8(6)9)16-17(11,13)14/h2-5H,1H3,(H2,11,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02220
BindingDB Entry DOI: 10.7270/Q20869C9 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513186
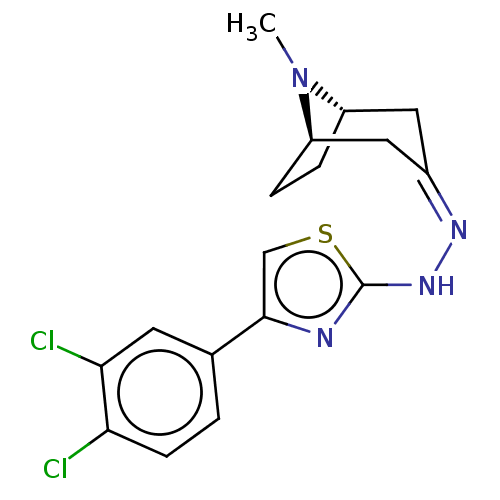
(CHEMBL4588381)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(Cl)c(Cl)c1)N2C |r,TLB:9:7:24:2.3| Show InChI InChI=1S/C17H18Cl2N4S/c1-23-12-3-4-13(23)8-11(7-12)21-22-17-20-16(9-24-17)10-2-5-14(18)15(19)6-10/h2,5-6,9,12-13H,3-4,7-8H2,1H3,(H,20,22)/b21-11-/t12-,13+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513187
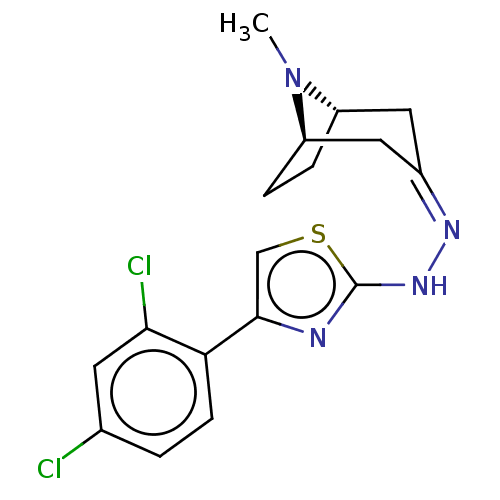
(CHEMBL4583240)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(Cl)cc1Cl)N2C |r,TLB:9:7:24:2.3| Show InChI InChI=1S/C17H18Cl2N4S/c1-23-12-3-4-13(23)8-11(7-12)21-22-17-20-16(9-24-17)14-5-2-10(18)6-15(14)19/h2,5-6,9,12-13H,3-4,7-8H2,1H3,(H,20,22)/b21-11-/t12-,13+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513185
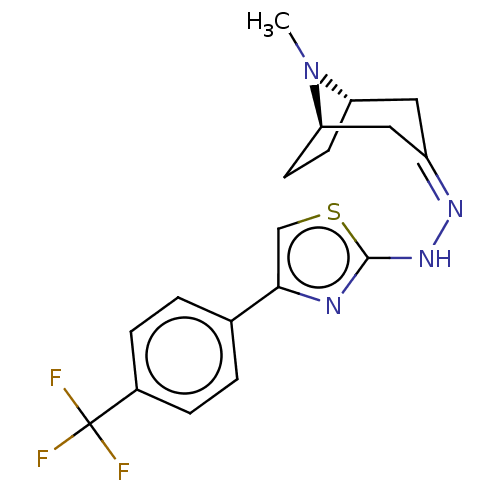
(CHEMBL4463818)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(cc1)C(F)(F)F)N2C |r,TLB:9:7:26:2.3| Show InChI InChI=1S/C18H19F3N4S/c1-25-14-6-7-15(25)9-13(8-14)23-24-17-22-16(10-26-17)11-2-4-12(5-3-11)18(19,20)21/h2-5,10,14-15H,6-9H2,1H3,(H,22,24)/b23-13-/t14-,15+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50031467

(5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...)Show InChI InChI=1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513188
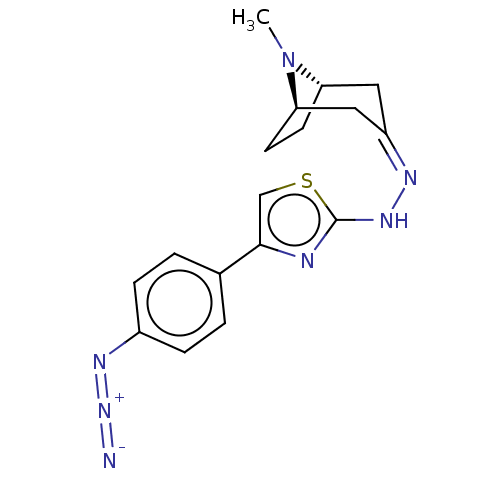
(CHEMBL4453112)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(cc1)N=[N+]=[N-])N2C |r,TLB:9:7:25:2.3| Show InChI InChI=1S/C17H19N7S/c1-24-14-6-7-15(24)9-13(8-14)20-22-17-19-16(10-25-17)11-2-4-12(5-3-11)21-23-18/h2-5,10,14-15H,6-9H2,1H3,(H,19,22)/b20-13-/t14-,15+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513183
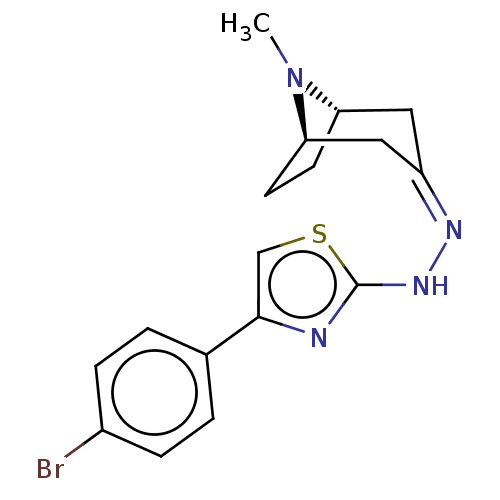
(CHEMBL4473661)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(Br)cc1)N2C |r,TLB:9:7:23:2.3| Show InChI InChI=1S/C17H19BrN4S/c1-22-14-6-7-15(22)9-13(8-14)20-21-17-19-16(10-23-17)11-2-4-12(18)5-3-11/h2-5,10,14-15H,6-9H2,1H3,(H,19,21)/b20-13-/t14-,15+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513190

(CHEMBL4438867)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(C)cc1)N2C |r,TLB:9:7:23:2.3| Show InChI InChI=1S/C18H22N4S/c1-12-3-5-13(6-4-12)17-11-23-18(19-17)21-20-14-9-15-7-8-16(10-14)22(15)2/h3-6,11,15-16H,7-10H2,1-2H3,(H,19,21)/b20-14-/t15-,16+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513189
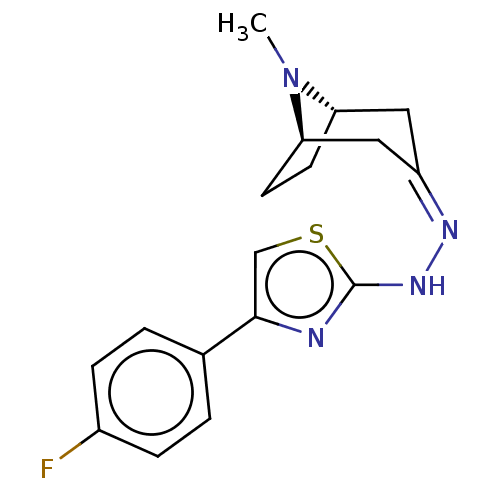
(CHEMBL4555931)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(F)cc1)N2C |r,TLB:9:7:23:2.3| Show InChI InChI=1S/C17H19FN4S/c1-22-14-6-7-15(22)9-13(8-14)20-21-17-19-16(10-23-17)11-2-4-12(18)5-3-11/h2-5,10,14-15H,6-9H2,1H3,(H,19,21)/b20-13-/t14-,15+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50513184
(CHEMBL4456453)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/Nc1nc(cs1)-c1ccc(Cl)cc1)N2C |r,TLB:9:7:23:2.3| Show InChI InChI=1S/C17H19ClN4S/c1-22-14-6-7-15(22)9-13(8-14)20-21-17-19-16(10-23-17)11-2-4-12(18)5-3-11/h2-5,10,14-15H,6-9H2,1H3,(H,19,21)/b20-13-/t14-,15+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50351096
(ASCORBIC ACID)Show InChI InChI=1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2-3,5,7-9H,1H2/t2-,3?,5+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nicolaus Copernicus University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-dopa as substrate incubated for 30 mins by spectrophotometric method |
Eur J Med Chem 175: 162-171 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.006
BindingDB Entry DOI: 10.7270/Q2794815 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data