

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50422387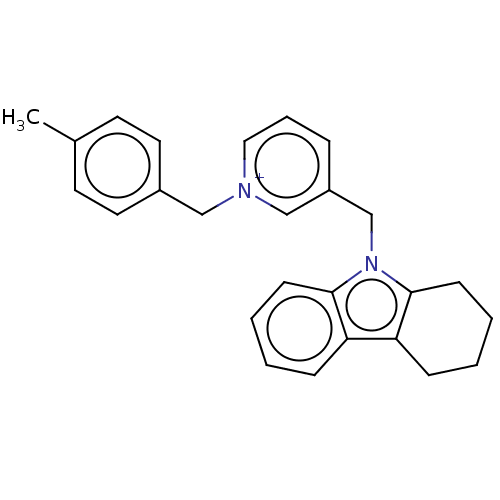 (CHEMBL4159171) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BChE using varying levels of butyrylthiocholine iodide as substrate by Lineweaver-burk plot analysis | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464028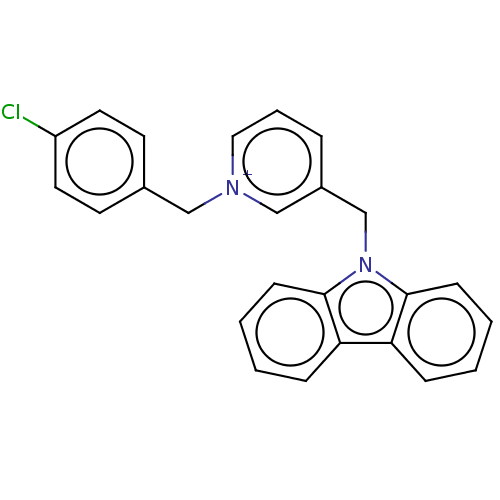 (CHEMBL4241164) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE using varying levels of butyrylthiocholine iodide as substrate preincubated for 10 mins followed by subst... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50268048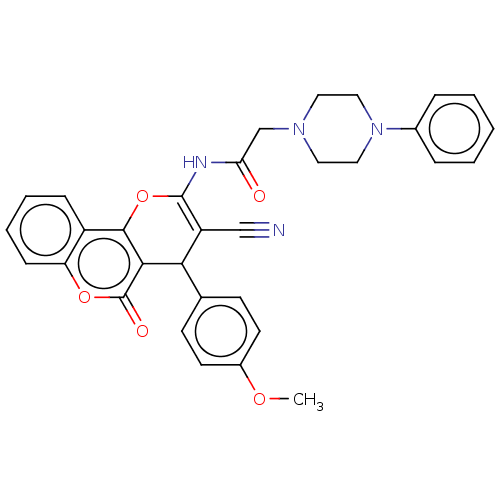 (CHEMBL4098491) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Faculty of Pharmacy, International Campus (TUMS-IC), Tehran University of Medical Sciences, Tehran, Iran. Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using varying levels of acetylthiocholine iodide as substrate measured for 2 mins by Lineweaver-Burk plot ... | Bioorg Med Chem 25: 3980-3988 (2017) Article DOI: 10.1016/j.bmc.2017.05.043 BindingDB Entry DOI: 10.7270/Q25Q4ZK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50422394 (CHEMBL3558149) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50189988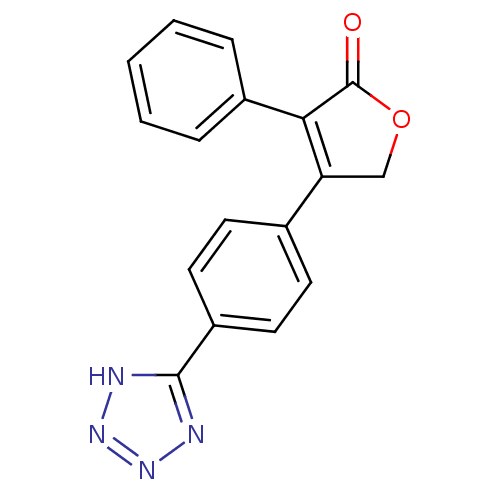 (CHEMBL214101 | Rofecoxib analogue) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX2 | Bioorg Med Chem Lett 16: 4483-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.032 BindingDB Entry DOI: 10.7270/Q2W095JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50189989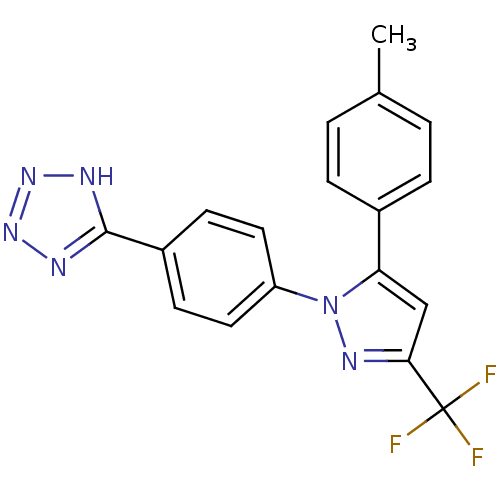 (4-(4-methylphenyl)-1-[4-(5-tetrazolyl)phenyl]-3-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX2 | Bioorg Med Chem Lett 16: 4483-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.032 BindingDB Entry DOI: 10.7270/Q2W095JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX2 | Bioorg Med Chem Lett 16: 4483-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.032 BindingDB Entry DOI: 10.7270/Q2W095JB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 (1 to 460 residues) expressed in baculovirus-infected insect cells using Rh-EVNLDAEFK-quencher as substrate mea... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Bos taurus) | BDBM50129504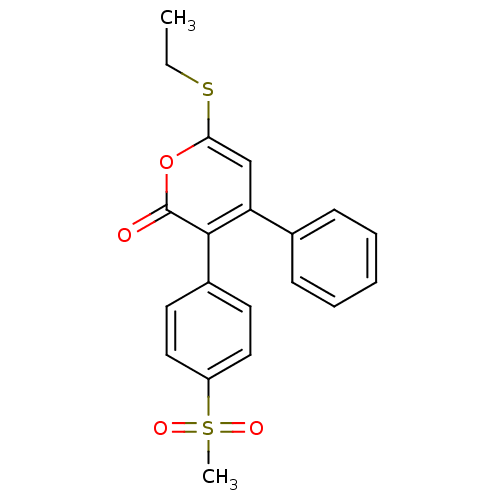 (6-Ethylsulfanyl-3-(4-methanesulfonyl-phenyl)-4-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 (COX-2) | J Med Chem 46: 4872-82 (2003) Article DOI: 10.1021/jm0302391 BindingDB Entry DOI: 10.7270/Q20V8DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50129504 (6-Ethylsulfanyl-3-(4-methanesulfonyl-phenyl)-4-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibition against ovine Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 13: 2205-9 (2003) BindingDB Entry DOI: 10.7270/Q2J102KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX1 | Bioorg Med Chem Lett 16: 4483-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.032 BindingDB Entry DOI: 10.7270/Q2W095JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50189988 (CHEMBL214101 | Rofecoxib analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX1 | Bioorg Med Chem Lett 16: 4483-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.032 BindingDB Entry DOI: 10.7270/Q2W095JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50189989 (4-(4-methylphenyl)-1-[4-(5-tetrazolyl)phenyl]-3-(t...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX1 | Bioorg Med Chem Lett 16: 4483-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.032 BindingDB Entry DOI: 10.7270/Q2W095JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM13065 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX1 in human blood assessed as PGE2 level incubated for 15 mins prior to LPS-challenge measured after 24 hrs by enzyme immunoassay | Bioorg Med Chem 21: 2355-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.058 BindingDB Entry DOI: 10.7270/Q2VT1TFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculty of Pharmacy, International Campus (TUMS-IC), Tehran University of Medical Sciences, Tehran, Iran. Curated by ChEMBL | Assay Description Inhibition of human BACE1 using Rh-EVNLDAEFK-quencher as substrate measured after 60 mins by FRET assay | Bioorg Med Chem 25: 3980-3988 (2017) Article DOI: 10.1016/j.bmc.2017.05.043 BindingDB Entry DOI: 10.7270/Q25Q4ZK4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured f... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 mi... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029600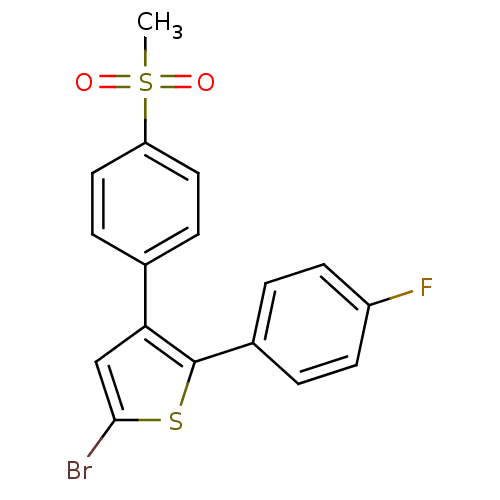 (5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX2 in human blood assessed as TxB2 level after 1 hr by enzyme immunoassay | Bioorg Med Chem 21: 2355-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.058 BindingDB Entry DOI: 10.7270/Q2VT1TFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculty of Pharmacy, International Campus (TUMS-IC), Tehran University of Medical Sciences, Tehran, Iran. Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured for 6 mins by Ellman's method | Bioorg Med Chem 25: 3980-3988 (2017) Article DOI: 10.1016/j.bmc.2017.05.043 BindingDB Entry DOI: 10.7270/Q25Q4ZK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10949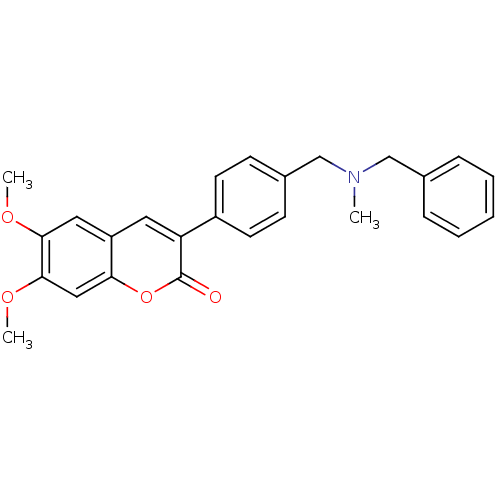 (3-(4-{[Benzyl(methyl)amino]methyl}-phenyl)-6,7-dim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human AChE | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10949 (3-(4-{[Benzyl(methyl)amino]methyl}-phenyl)-6,7-dim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculty of Pharmacy, International Campus (TUMS-IC), Tehran University of Medical Sciences, Tehran, Iran. Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine as substrate by Ellman's method | Bioorg Med Chem 25: 3980-3988 (2017) Article DOI: 10.1016/j.bmc.2017.05.043 BindingDB Entry DOI: 10.7270/Q25Q4ZK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50432281 (CHEMBL2347671) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX2 in human blood assessed as TxB2 level after 1 hr by enzyme immunoassay | Bioorg Med Chem 21: 2355-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.058 BindingDB Entry DOI: 10.7270/Q2VT1TFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Bos taurus) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 (COX-2) | J Med Chem 46: 4872-82 (2003) Article DOI: 10.1021/jm0302391 BindingDB Entry DOI: 10.7270/Q20V8DHZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibition against ovine Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 13: 2205-9 (2003) BindingDB Entry DOI: 10.7270/Q2J102KC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate by chemiluminescence assay | Bioorg Med Chem 22: 865-73 (2014) Article DOI: 10.1016/j.bmc.2013.12.002 BindingDB Entry DOI: 10.7270/Q2319ZV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50432287 (CHEMBL2347677) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX2 in human blood assessed as TxB2 level after 1 hr by enzyme immunoassay | Bioorg Med Chem 21: 2355-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.058 BindingDB Entry DOI: 10.7270/Q2VT1TFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50432286 (CHEMBL2347667) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX2 in human blood assessed as TxB2 level after 1 hr by enzyme immunoassay | Bioorg Med Chem 21: 2355-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.058 BindingDB Entry DOI: 10.7270/Q2VT1TFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464028 (CHEMBL4241164) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50432280 (CHEMBL2347672) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX2 in human blood assessed as TxB2 level after 1 hr by enzyme immunoassay | Bioorg Med Chem 21: 2355-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.058 BindingDB Entry DOI: 10.7270/Q2VT1TFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50422387 (CHEMBL4159171) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Bos taurus) | BDBM50134831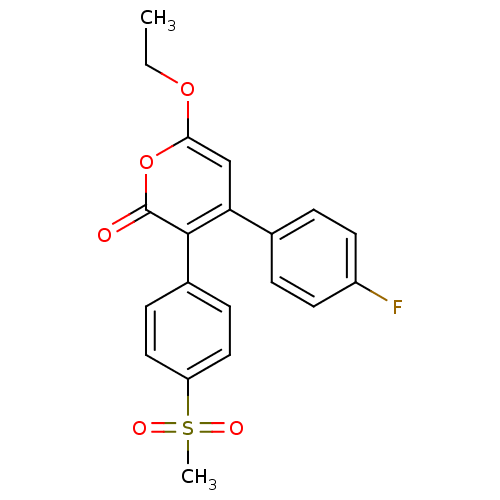 (6-Ethoxy-4-(4-fluoro-phenyl)-3-(4-methanesulfonyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description In vitro inhibitory activity against prostaglandin G/H synthase 2 (COX-2) | J Med Chem 46: 4872-82 (2003) Article DOI: 10.1021/jm0302391 BindingDB Entry DOI: 10.7270/Q20V8DHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50495225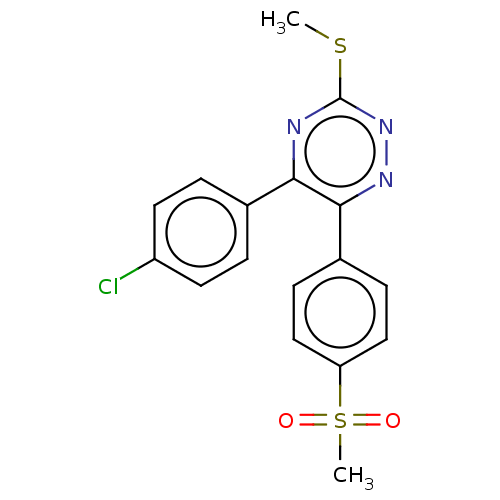 (CHEMBL3103780) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate by chemiluminescence assay | Bioorg Med Chem 22: 865-73 (2014) Article DOI: 10.1016/j.bmc.2013.12.002 BindingDB Entry DOI: 10.7270/Q2319ZV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50432285 (CHEMBL2347668) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX2 in human blood assessed as TxB2 level after 1 hr by enzyme immunoassay | Bioorg Med Chem 21: 2355-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.058 BindingDB Entry DOI: 10.7270/Q2VT1TFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464019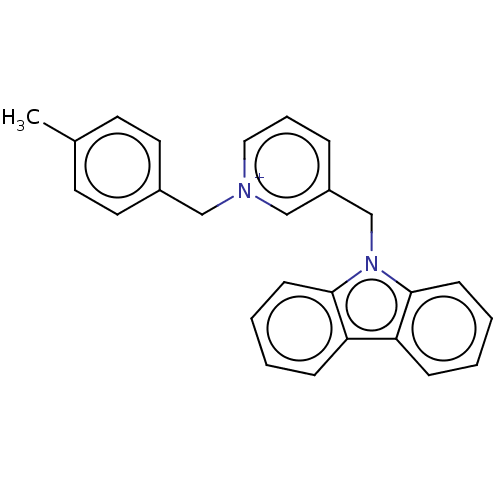 (CHEMBL4245106) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50432279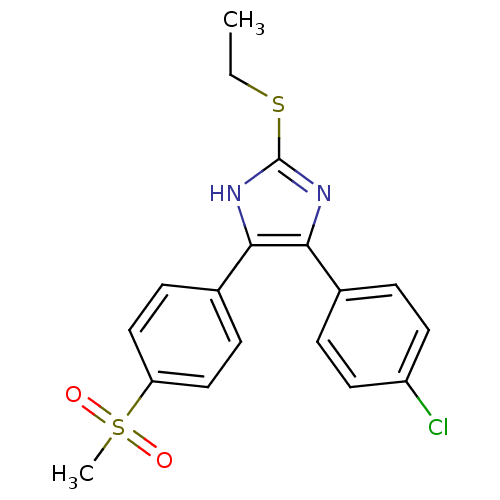 (CHEMBL2347673) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX2 in human blood assessed as TxB2 level after 1 hr by enzyme immunoassay | Bioorg Med Chem 21: 2355-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.058 BindingDB Entry DOI: 10.7270/Q2VT1TFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50422386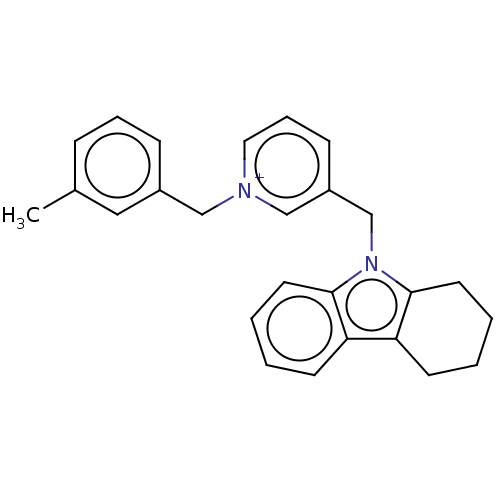 (CHEMBL4169820) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464031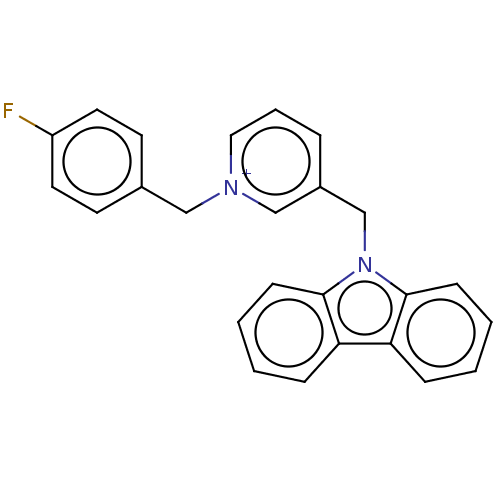 (CHEMBL4248896) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50495224 (CHEMBL3103781) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate by chemiluminescence assay | Bioorg Med Chem 22: 865-73 (2014) Article DOI: 10.1016/j.bmc.2013.12.002 BindingDB Entry DOI: 10.7270/Q2319ZV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464025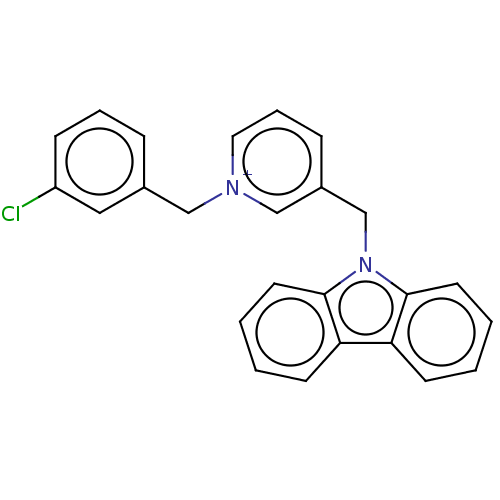 (CHEMBL4244170) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50432287 (CHEMBL2347677) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX1 in human blood assessed as PGE2 level incubated for 15 mins prior to LPS-challenge measured after 24 hrs by enzyme immunoassay | Bioorg Med Chem 21: 2355-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.058 BindingDB Entry DOI: 10.7270/Q2VT1TFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50432281 (CHEMBL2347671) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX1 in human blood assessed as PGE2 level incubated for 15 mins prior to LPS-challenge measured after 24 hrs by enzyme immunoassay | Bioorg Med Chem 21: 2355-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.058 BindingDB Entry DOI: 10.7270/Q2VT1TFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50495223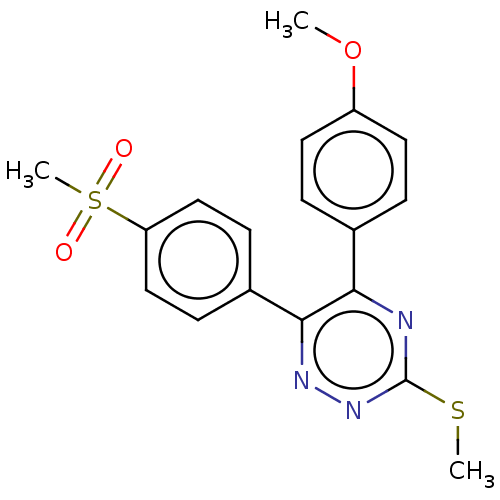 (CHEMBL3103779) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate by chemiluminescence assay | Bioorg Med Chem 22: 865-73 (2014) Article DOI: 10.1016/j.bmc.2013.12.002 BindingDB Entry DOI: 10.7270/Q2319ZV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50495222 (CHEMBL3103782) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate by chemiluminescence assay | Bioorg Med Chem 22: 865-73 (2014) Article DOI: 10.1016/j.bmc.2013.12.002 BindingDB Entry DOI: 10.7270/Q2319ZV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50495226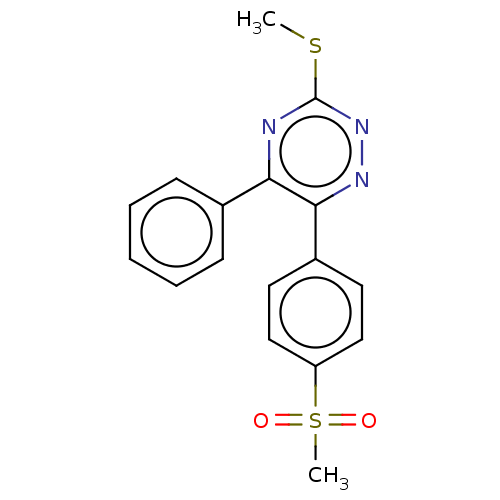 (CHEMBL3103783) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Mazandaran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate by chemiluminescence assay | Bioorg Med Chem 22: 865-73 (2014) Article DOI: 10.1016/j.bmc.2013.12.002 BindingDB Entry DOI: 10.7270/Q2319ZV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421756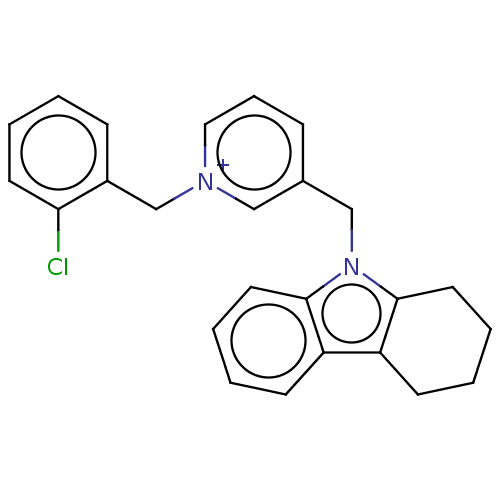 (CHEMBL4161385) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of ovine COX1 | Bioorg Med Chem 15: 1976-82 (2007) Article DOI: 10.1016/j.bmc.2006.12.041 BindingDB Entry DOI: 10.7270/Q2S75FZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50432286 (CHEMBL2347667) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX1 in human blood assessed as PGE2 level incubated for 15 mins prior to LPS-challenge measured after 24 hrs by enzyme immunoassay | Bioorg Med Chem 21: 2355-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.058 BindingDB Entry DOI: 10.7270/Q2VT1TFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464021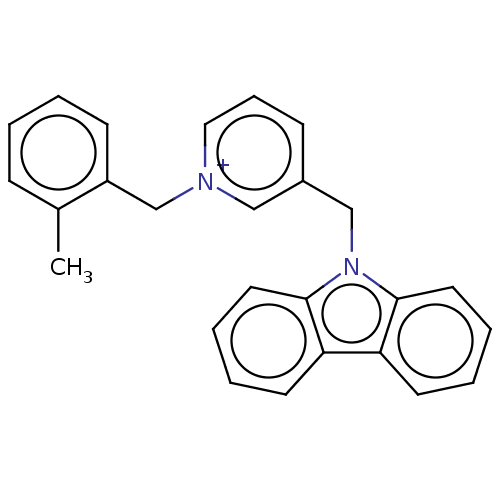 (CHEMBL4241338) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421747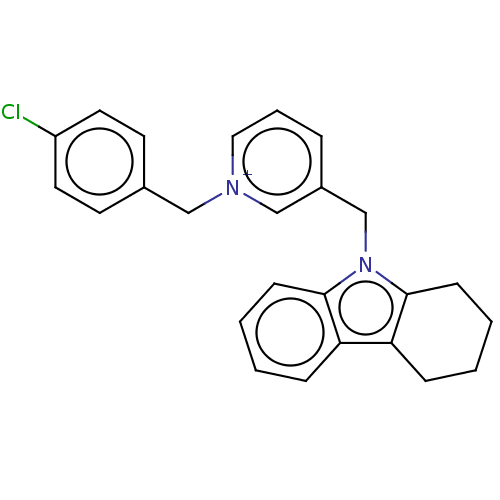 (CHEMBL4171576) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50432280 (CHEMBL2347672) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX1 in human blood assessed as PGE2 level incubated for 15 mins prior to LPS-challenge measured after 24 hrs by enzyme immunoassay | Bioorg Med Chem 21: 2355-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.058 BindingDB Entry DOI: 10.7270/Q2VT1TFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 270 total ) | Next | Last >> |