

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50100134 (2-(4-Dimethylamino-phenyl)-3,6-dimethyl-benzothiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [N-methyl-11C]6-Me-BTA-1 from pre-aggregated amyloid beta (1 to 40) fibrils (unknown origin) after 30 mins by scintillation counting ... | J Nat Prod 80: 278-289 (2017) Article DOI: 10.1021/acs.jnatprod.6b00643 BindingDB Entry DOI: 10.7270/Q2CR5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50140172 (CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) aggregation after 1 hr by thioflavin-T fluorescence assay | J Nat Prod 80: 278-289 (2017) Article DOI: 10.1021/acs.jnatprod.6b00643 BindingDB Entry DOI: 10.7270/Q2CR5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50139772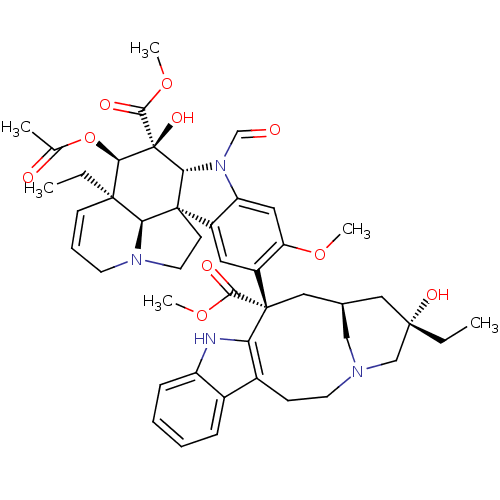 (22-oxovincaleukoblastine | leurocristine | vincris...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS Curated by ChEMBL | Assay Description In vitro inhibition of tubulin polymerization measured as turbidity formed by centrifugation | J Med Chem 41: 1524-30 (1998) Article DOI: 10.1021/jm970800t BindingDB Entry DOI: 10.7270/Q2MW2KV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50216333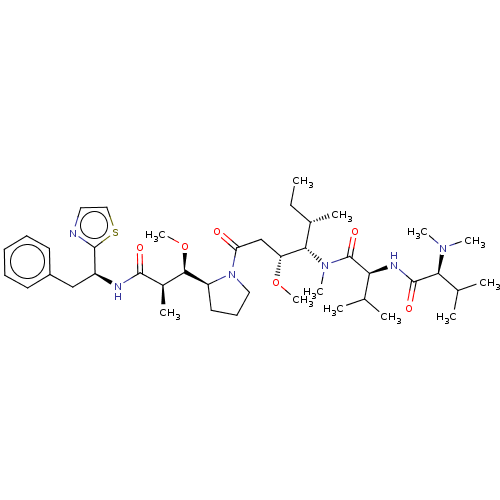 (CHEBI:67357 | DOLASTATIN-10 | Dolastatin 10 | Dolo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire des M£canismes Mol£culaires des Communications Cellulaires (CNRS UPR 9023) Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of tubulin polymerization. | Bioorg Med Chem Lett 8: 2855-8 (1999) BindingDB Entry DOI: 10.7270/Q2H41RX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50216333 (CHEBI:67357 | DOLASTATIN-10 | Dolastatin 10 | Dolo...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS Curated by ChEMBL | Assay Description In vitro inhibition of tubulin polymerization measured as turbidity formed by centrifugation | J Med Chem 41: 1524-30 (1998) Article DOI: 10.1021/jm970800t BindingDB Entry DOI: 10.7270/Q2MW2KV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50472222 (CHEMBL608244) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS Curated by ChEMBL | Assay Description In vitro inhibition of tubulin polymerization measured as turbidity formed by centrifugation | J Med Chem 41: 1524-30 (1998) Article DOI: 10.1021/jm970800t BindingDB Entry DOI: 10.7270/Q2MW2KV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50140172 (CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 42) aggregation after 46 to 48 hrs by Thioflavin T fluorescence assay | J Nat Prod 80: 278-289 (2017) Article DOI: 10.1021/acs.jnatprod.6b00643 BindingDB Entry DOI: 10.7270/Q2CR5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM212434 ((2R,3R)-3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of amyloid beta (1 to 42) (unknown origin) aggregation by Thioflavin-T fluorescence assay | J Nat Prod 80: 278-289 (2017) Article DOI: 10.1021/acs.jnatprod.6b00643 BindingDB Entry DOI: 10.7270/Q2CR5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50472221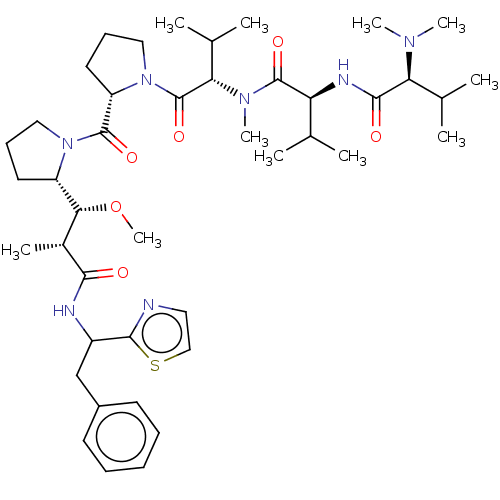 (CHEMBL37030) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS Curated by ChEMBL | Assay Description In vitro inhibition of tubulin polymerization measured as turbidity formed by centrifugation | J Med Chem 41: 1524-30 (1998) Article DOI: 10.1021/jm970800t BindingDB Entry DOI: 10.7270/Q2MW2KV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of amyloid beta (1 to 42) (unknown origin) aggregation by Thioflavin-T fluorescence assay | J Nat Prod 80: 278-289 (2017) Article DOI: 10.1021/acs.jnatprod.6b00643 BindingDB Entry DOI: 10.7270/Q2CR5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM50472220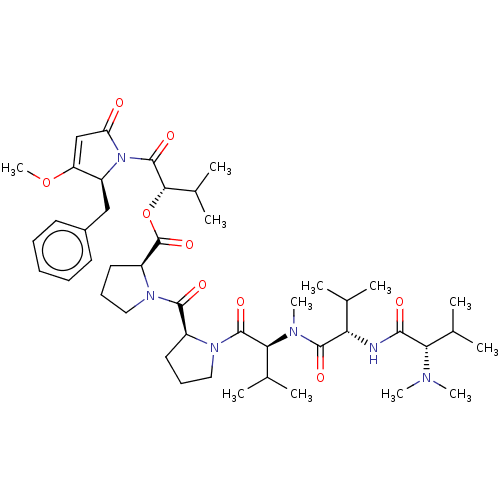 (Dolastatin 15 | Dolostatin 15) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UPR 9023 CNRS Curated by ChEMBL | Assay Description In vitro inhibition of tubulin polymerization measured as turbidity formed by centrifugation | J Med Chem 41: 1524-30 (1998) Article DOI: 10.1021/jm970800t BindingDB Entry DOI: 10.7270/Q2MW2KV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant amyloid beta (1 to 42) fibrils (unknown origin) by thioflavin-T fluorescence assay | J Nat Prod 80: 278-289 (2017) Article DOI: 10.1021/acs.jnatprod.6b00643 BindingDB Entry DOI: 10.7270/Q2CR5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50072182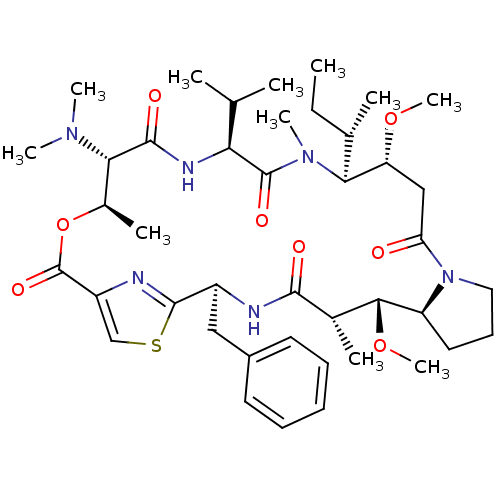 ((2S,5S,6R,7S,14R,15S,18S,21S,22R)-2-Benzyl-15-((S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire des M£canismes Mol£culaires des Communications Cellulaires (CNRS UPR 9023) Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibition of tubulin polymerization | Bioorg Med Chem Lett 8: 2855-8 (1999) BindingDB Entry DOI: 10.7270/Q2H41RX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of amyloid beta (1 to 42) (unknown origin) aggregation by Thioflavin-T fluorescence assay | J Nat Prod 80: 278-289 (2017) Article DOI: 10.1021/acs.jnatprod.6b00643 BindingDB Entry DOI: 10.7270/Q2CR5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of amyloid beta (1 to 42) (unknown origin) aggregation by Thioflavin-T fluorescence assay | J Nat Prod 80: 278-289 (2017) Article DOI: 10.1021/acs.jnatprod.6b00643 BindingDB Entry DOI: 10.7270/Q2CR5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of amyloid beta (1 to 42) (unknown origin) aggregation by Thioflavin-T fluorescence assay | J Nat Prod 80: 278-289 (2017) Article DOI: 10.1021/acs.jnatprod.6b00643 BindingDB Entry DOI: 10.7270/Q2CR5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50240348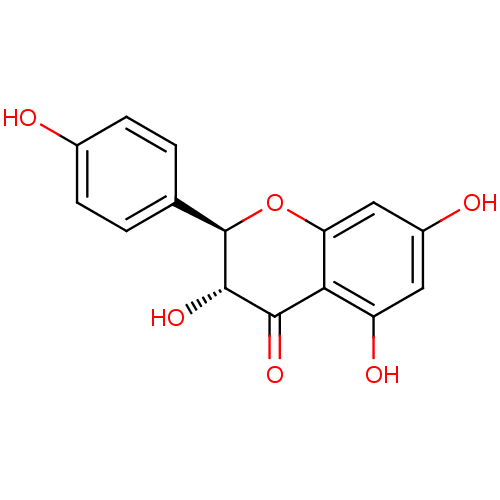 ((+)-dihydrokaempferol | (2R,3R)-3,5,7-trihydroxy-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of amyloid beta (1 to 42) (unknown origin) aggregation by Thioflavin-T fluorescence assay | J Nat Prod 80: 278-289 (2017) Article DOI: 10.1021/acs.jnatprod.6b00643 BindingDB Entry DOI: 10.7270/Q2CR5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50481119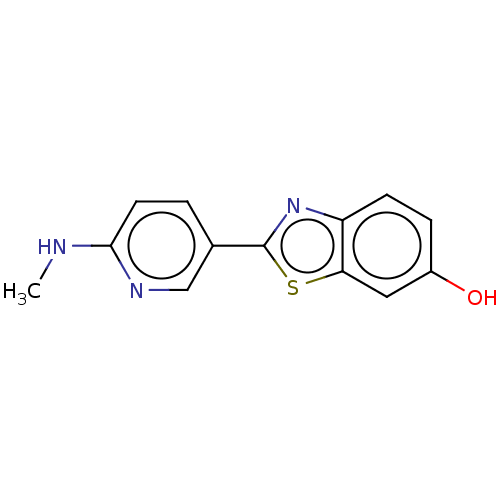 (AZD-2184 | CHEMBL570378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]PIB from human amyloid beta (1 to 40) fibrils after 3 hrs by liquid scintillation counting | J Nat Prod 80: 278-289 (2017) Article DOI: 10.1021/acs.jnatprod.6b00643 BindingDB Entry DOI: 10.7270/Q2CR5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||