

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM642921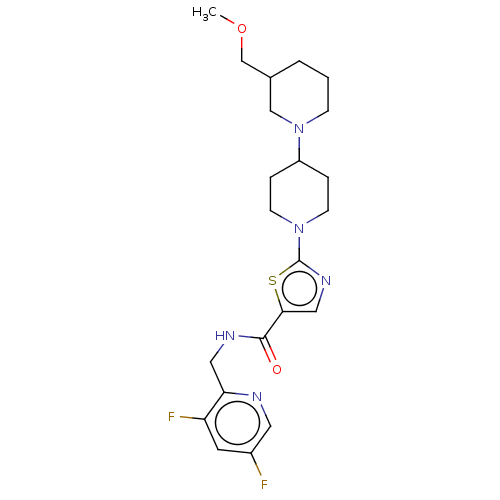 (US20240000767, Example 100 | US20240000767, Exampl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086170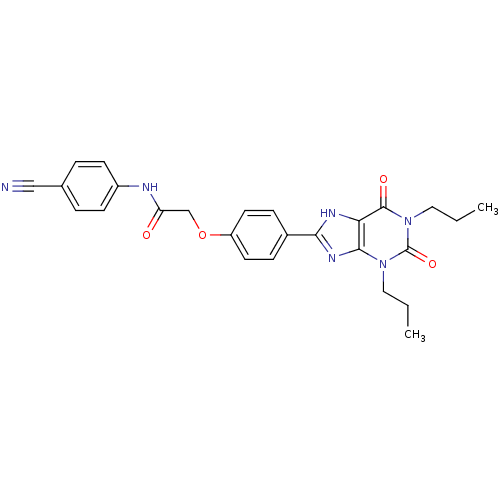 ((4-Cyano-phenyl)-carbamic acid 4-(2,6-dioxo-1,3-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human A2B adenosine receptor | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM415448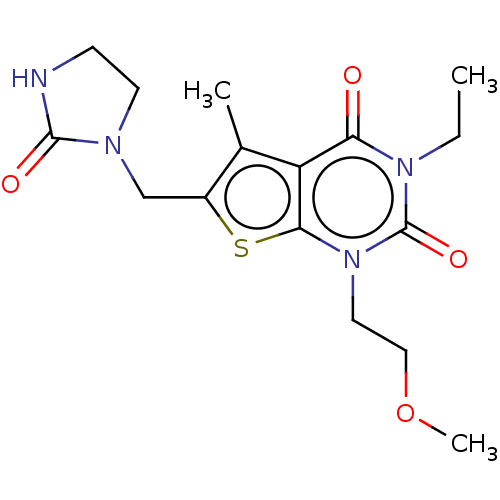 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoimida...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM415450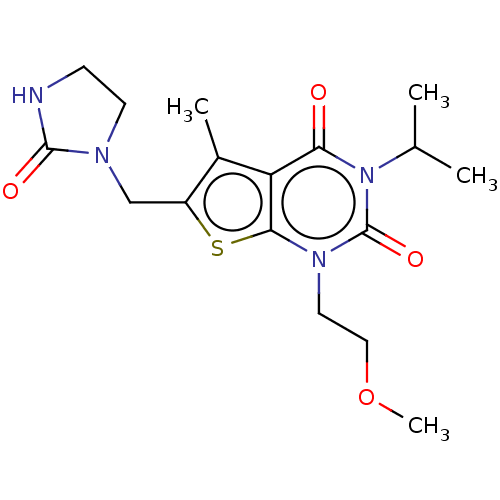 (3-Isopropyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM415451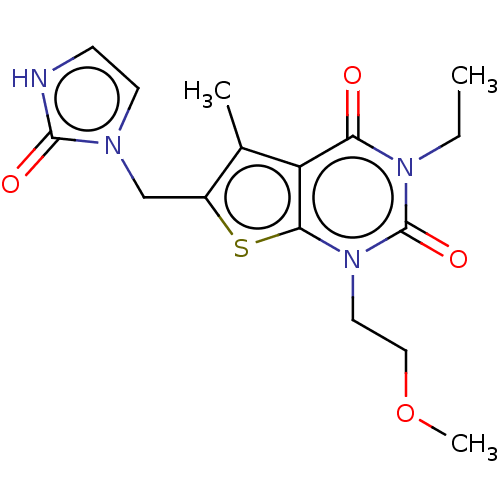 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxo-2,3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM415449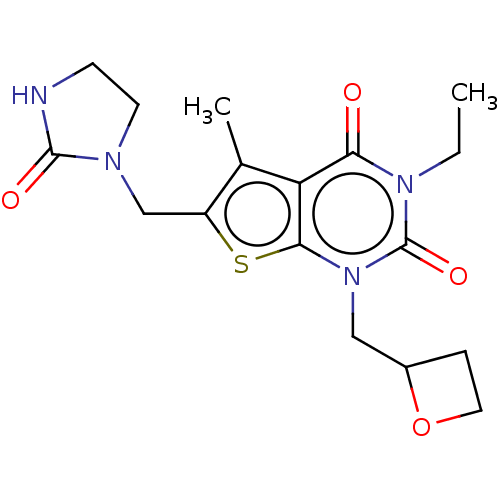 (3-Ethyl-5-methyl-1-(oxetan-2-ylmethyl)-6-[(2-oxoim...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM415452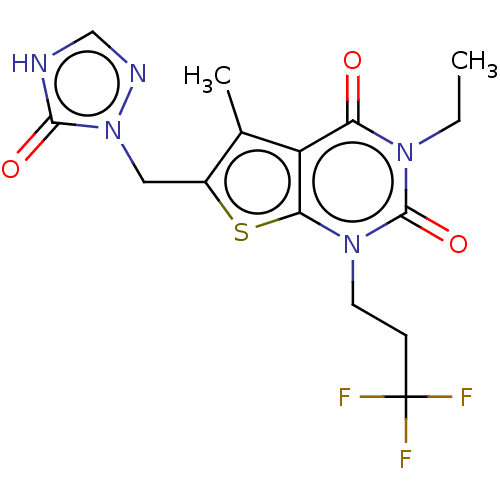 (3-Ethyl-5-methyl-6-[(5-oxo-4,5-dihydro-1H-1,2,4-tr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM415453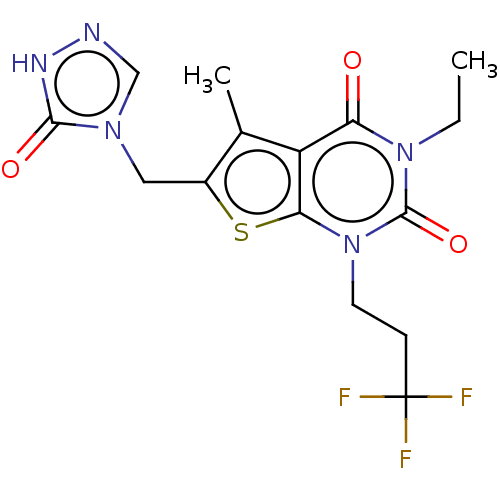 (3-Ethyl-5-methyl-6-[(5-oxo-1,5-dihydro-4H-1,2,4-tr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM642917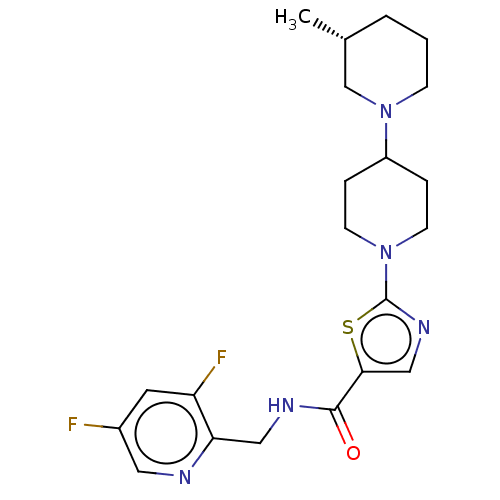 (N-[(3,5-Difluoropyridin-2-yl)methyl]-2-[(3R)-3-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50336977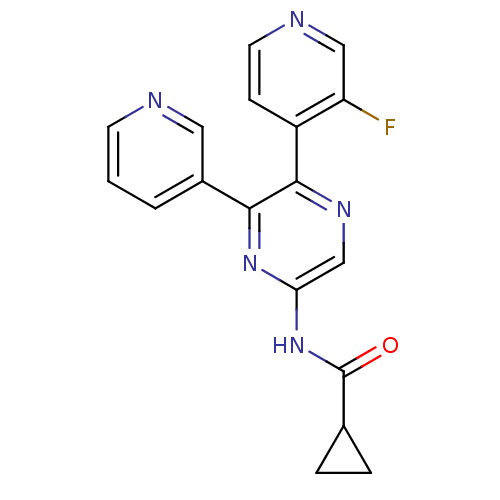 (CHEMBL1672627 | N-[5-(3-Fluoropyridin-4-yl)-6-pyri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human A2B adenosine receptor | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM415451 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxo-2,3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM415448 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoimida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM415450 (3-Isopropyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM415449 (3-Ethyl-5-methyl-1-(oxetan-2-ylmethyl)-6-[(2-oxoim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM415448 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM415453 (3-Ethyl-5-methyl-6-[(5-oxo-1,5-dihydro-4H-1,2,4-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM415451 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxo-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM415453 (3-Ethyl-5-methyl-6-[(5-oxo-1,5-dihydro-4H-1,2,4-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM415449 (3-Ethyl-5-methyl-1-(oxetan-2-ylmethyl)-6-[(2-oxoim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50531422 (CHEMBL4522981) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human A2A adenosine receptor expressed in HEK293 cell membranes after 120 mins by radioligand displacement assay | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50531422 (CHEMBL4522981) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins by radioligand displacement assay | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM415450 (3-Isopropyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM415452 (3-Ethyl-5-methyl-6-[(5-oxo-4,5-dihydro-1H-1,2,4-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM415452 (3-Ethyl-5-methyl-6-[(5-oxo-4,5-dihydro-1H-1,2,4-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM415453 (3-Ethyl-5-methyl-6-[(5-oxo-1,5-dihydro-4H-1,2,4-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM415449 (3-Ethyl-5-methyl-1-(oxetan-2-ylmethyl)-6-[(2-oxoim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM10849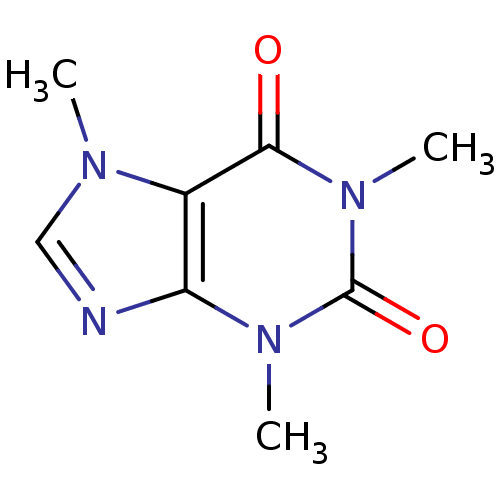 (1,3,7-trimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human A2B adenosine receptor | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50531422 (CHEMBL4522981) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human A3 adenosine receptor expressed in HEK293 cell membranes after 120 mins by radioligand displacement assay | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM415448 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM415451 (3-Ethyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxo-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM415450 (3-Isopropyl-1-(2-methoxyethyl)-5-methyl-6-[(2-oxoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The binding properties of the test compounds on adenosine receptors were determined in binding studies with radioligands. For this purpose, membrane ... | US Patent US10428083 (2019) BindingDB Entry DOI: 10.7270/Q2Z03BH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50574215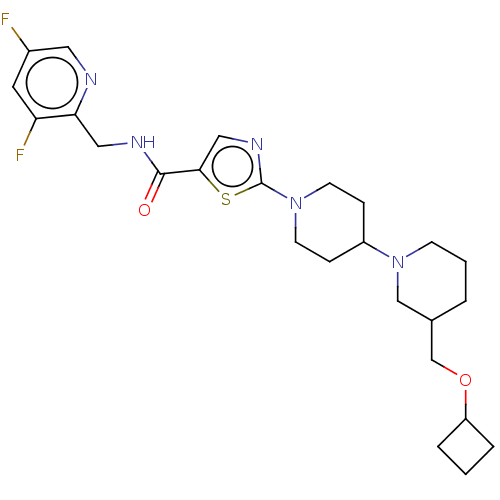 (CHEMBL4878875 | US20240000767, Example 102) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50574218 (CHEMBL4875484 | US20240000767, Example 135) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM104826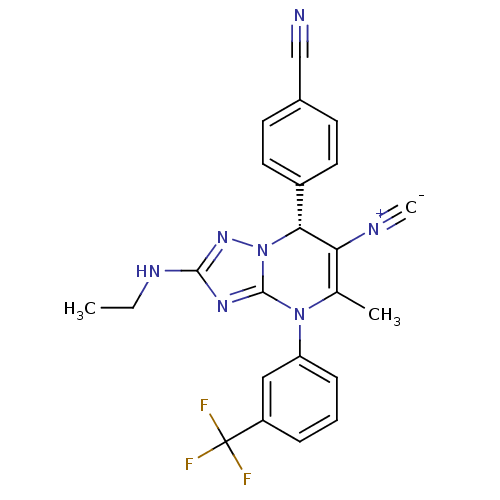 (US8569314, 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido... | US Patent US8569314 (2013) BindingDB Entry DOI: 10.7270/Q2FJ2FF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189921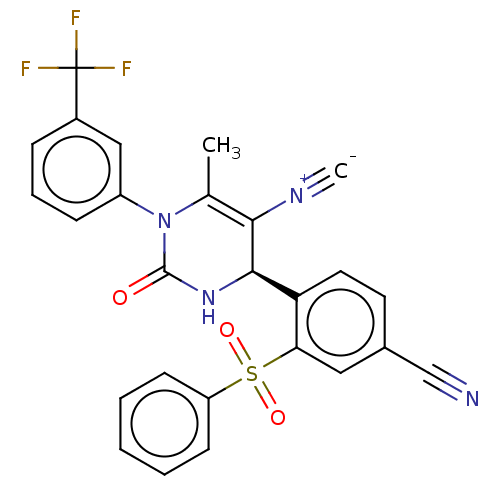 (US9174997, 141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189908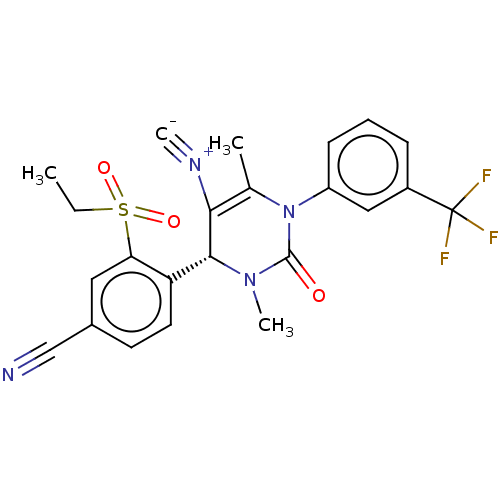 (US9174997, 128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189900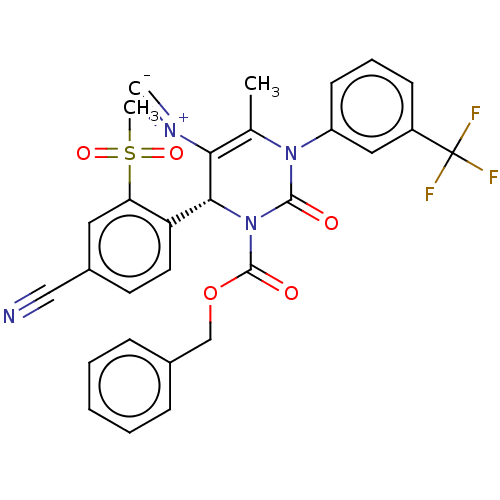 (US9174997, 120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189896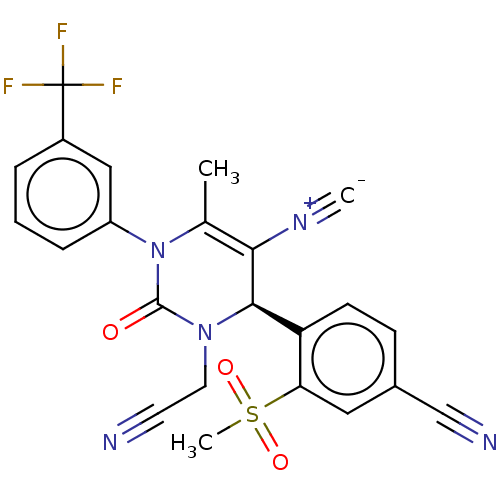 (US9174997, 116) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189883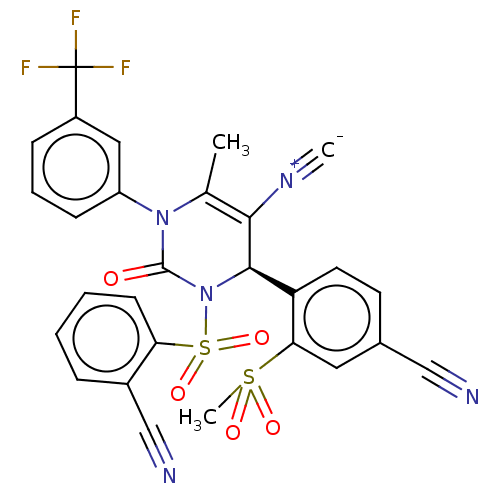 (US9174997, 103) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189876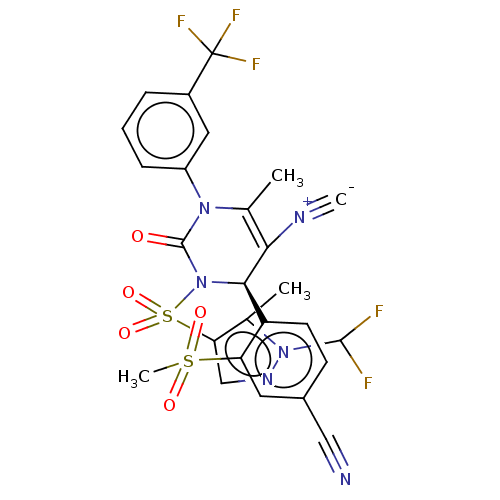 (US9174997, 96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189871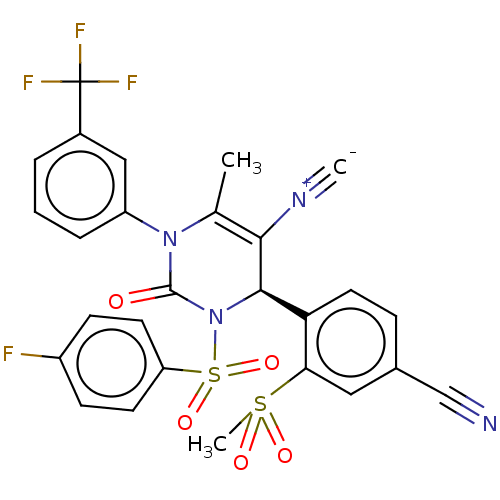 (US9174997, 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189865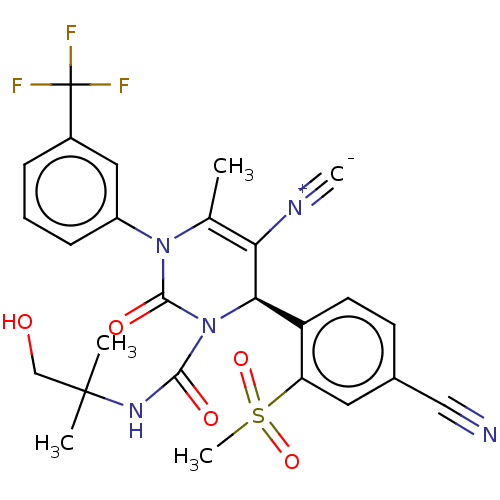 (US9174997, 85) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189860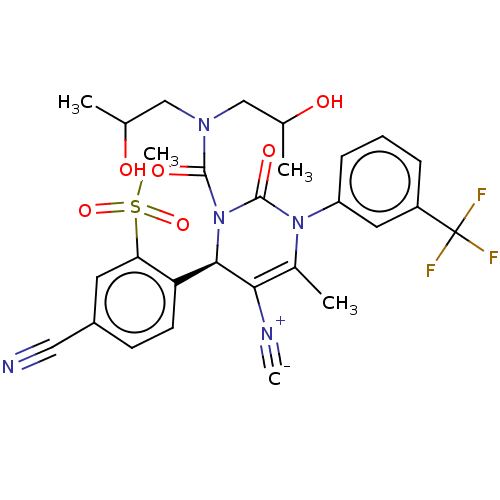 (US9174997, 80) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189835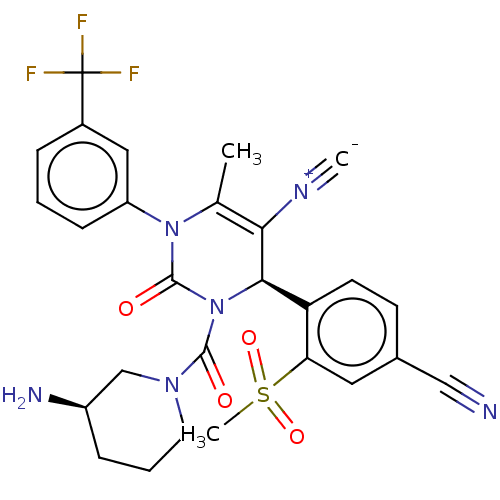 (US9174997, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189831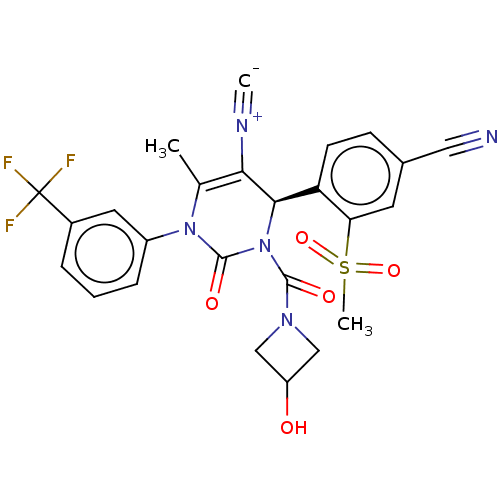 (US9174997, 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189823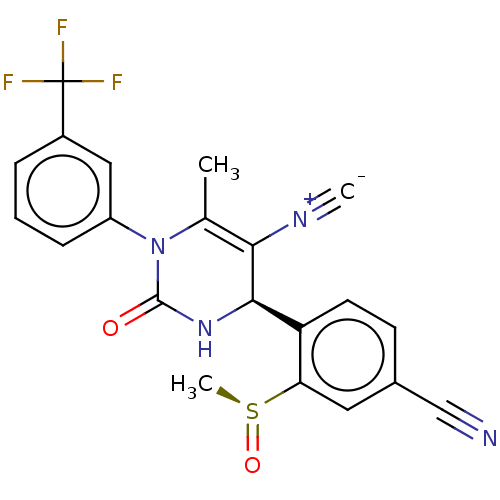 (US9174997, 43 (Diastereomer 1)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189822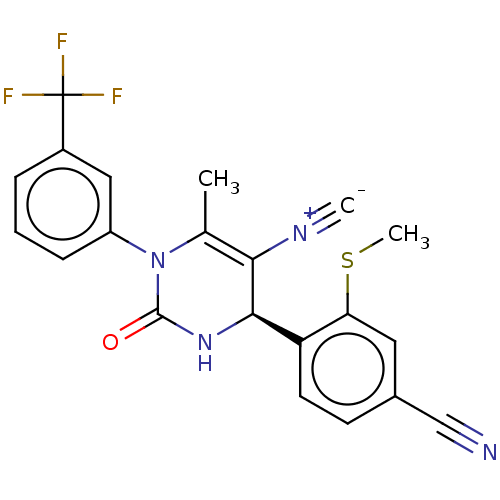 (US9174997, 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189821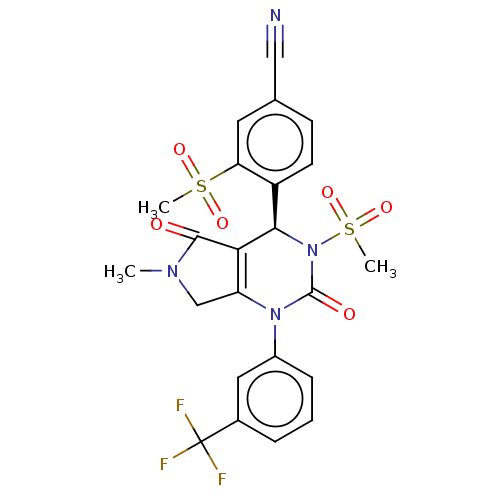 (US9174997, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189820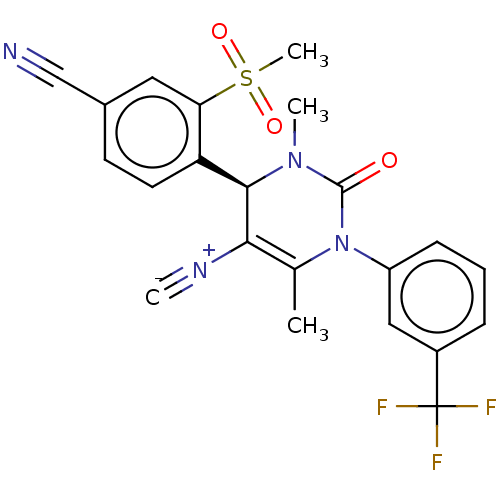 (US9174997, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189819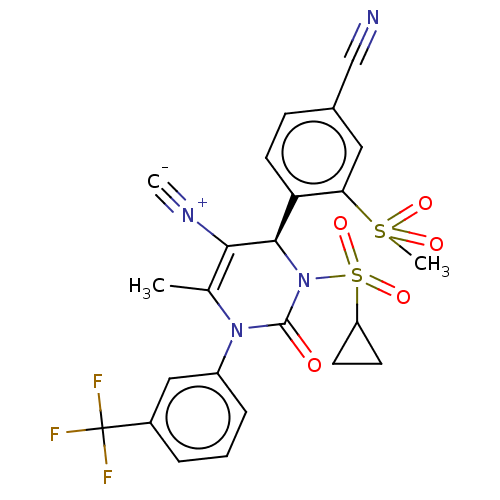 (US9174997, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1290 total ) | Next | Last >> |