

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457028 (CHEMBL4218303) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measur... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457026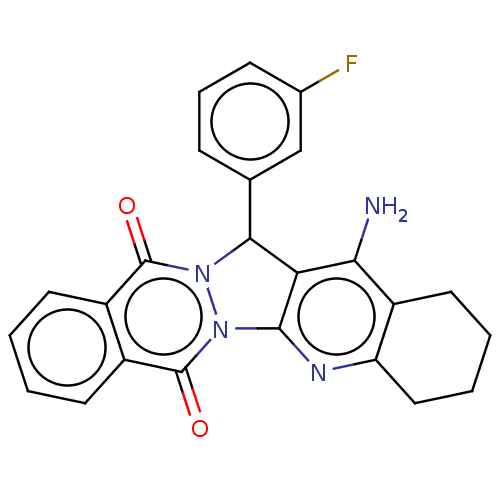 (CHEMBL4207023) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457028 (CHEMBL4218303) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457015 (CHEMBL4203173) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457024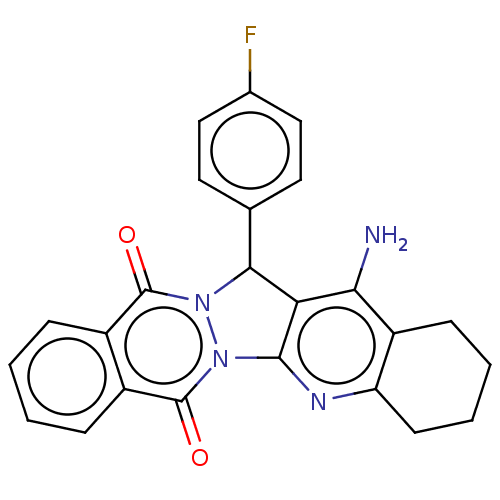 (CHEMBL4207926) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457014 (CHEMBL4203040) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457023 (CHEMBL4207397) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457025 (CHEMBL4210751) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457030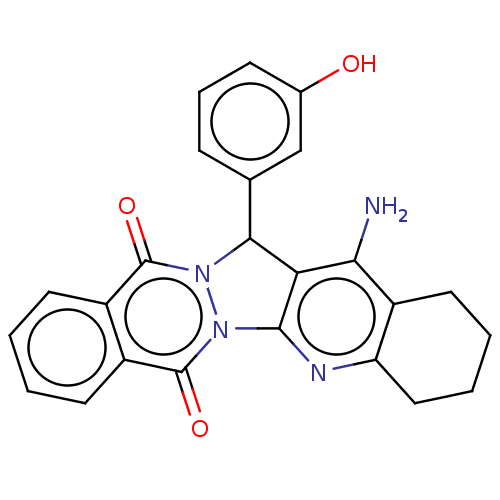 (CHEMBL4206121) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457033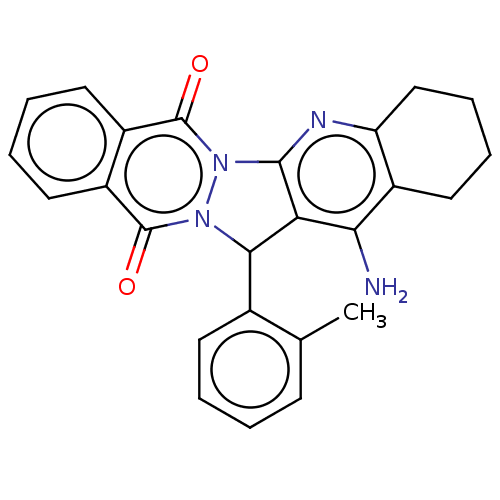 (CHEMBL4204269) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457027 (CHEMBL4203909) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457029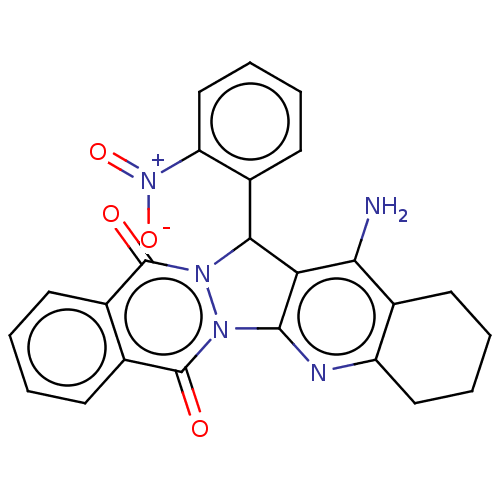 (CHEMBL4209859) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457032 (CHEMBL4212290) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 365 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457013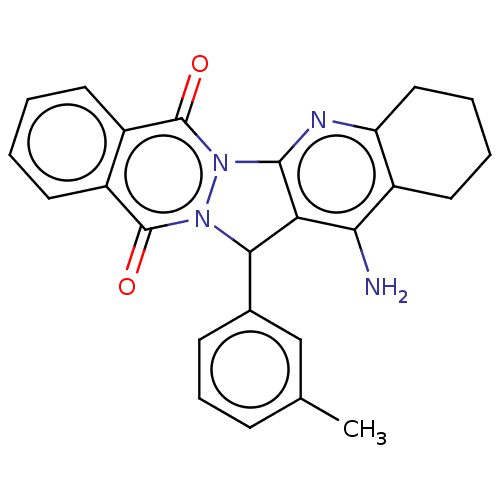 (CHEMBL4213928) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 467 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457022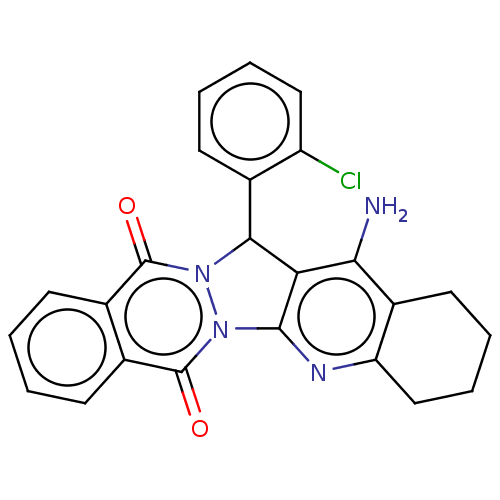 (CHEMBL4208973) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 592 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457016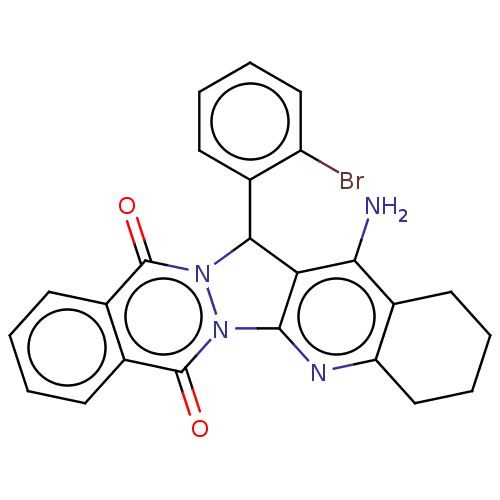 (CHEMBL4210767) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 599 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457021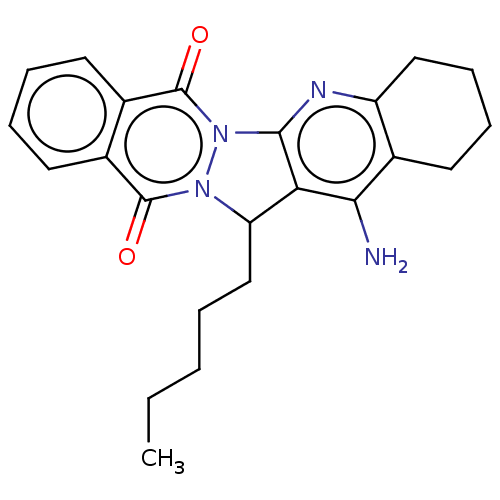 (CHEMBL4202878) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 612 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457020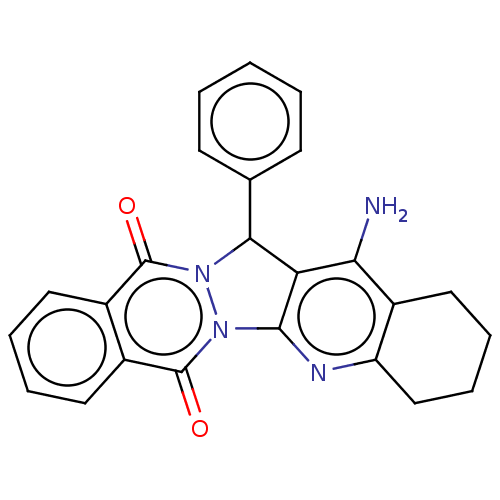 (CHEMBL4216504) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 614 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457019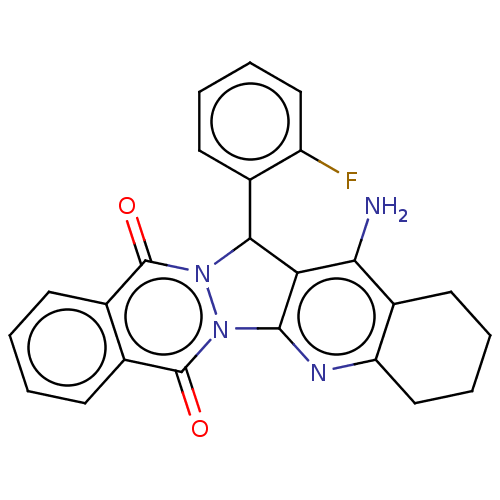 (CHEMBL4207471) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 618 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457018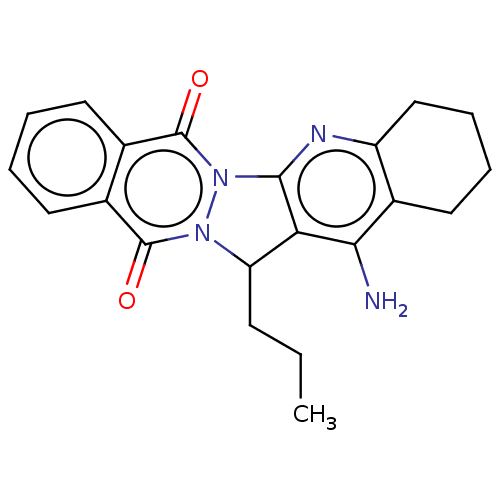 (CHEMBL4211075) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457017 (CHEMBL4208227) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50457031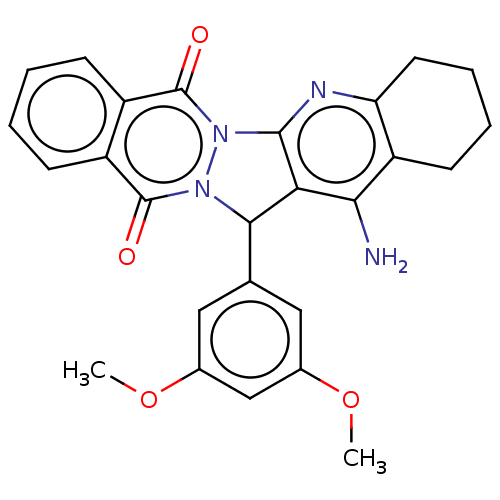 (CHEMBL4216769) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457026 (CHEMBL4207023) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457028 (CHEMBL4218303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457015 (CHEMBL4203173) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457025 (CHEMBL4210751) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457014 (CHEMBL4203040) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457023 (CHEMBL4207397) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457021 (CHEMBL4202878) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457027 (CHEMBL4203909) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457029 (CHEMBL4209859) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457033 (CHEMBL4204269) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457013 (CHEMBL4213928) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457024 (CHEMBL4207926) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457022 (CHEMBL4208973) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457016 (CHEMBL4210767) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457031 (CHEMBL4216769) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457030 (CHEMBL4206121) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457017 (CHEMBL4208227) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457018 (CHEMBL4211075) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457019 (CHEMBL4207471) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457020 (CHEMBL4216504) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50457032 (CHEMBL4212290) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 ... | Eur J Med Chem 139: 280-289 (2017) Article DOI: 10.1016/j.ejmech.2017.07.072 BindingDB Entry DOI: 10.7270/Q2SX6GVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||