 Found 395 hits with Last Name = 'hornberger' and Initial = 'm'
Found 395 hits with Last Name = 'hornberger' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM427470

(US10538497, Example 3)Show SMILES NS(=O)(=O)NCCSc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H12BrFN6O4S2/c12-7-5-6(1-2-8(7)13)16-10(17-20)9-11(19-23-18-9)24-4-3-15-25(14,21)22/h1-2,5,15,20H,3-4H2,(H,16,17)(H2,14,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549535

(CHEMBL4763375)Show SMILES [H][C@@]1(CC[C@@]2(COc3cc(ccc23)C(F)(F)F)CC1)[C@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:4.4,19.22,wD:1.0,(49.52,-49.21,;49.47,-50.71,;50.8,-49.93,;52.14,-50.7,;52.14,-52.25,;53.43,-51.41,;54.62,-52.38,;54.07,-53.81,;54.76,-55.17,;53.93,-56.45,;52.39,-56.37,;51.7,-55,;52.54,-53.72,;54.62,-57.81,;55.95,-57.04,;55.96,-58.58,;53.79,-59.11,;50.8,-53.03,;49.47,-52.25,;48.13,-49.95,;48.12,-48.41,;46.79,-50.72,;46.79,-52.27,;45.45,-49.96,;44.12,-50.73,;44.12,-52.29,;42.78,-53.07,;41.44,-52.29,;40.1,-53.06,;41.44,-50.74,;42.77,-49.97,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50285416
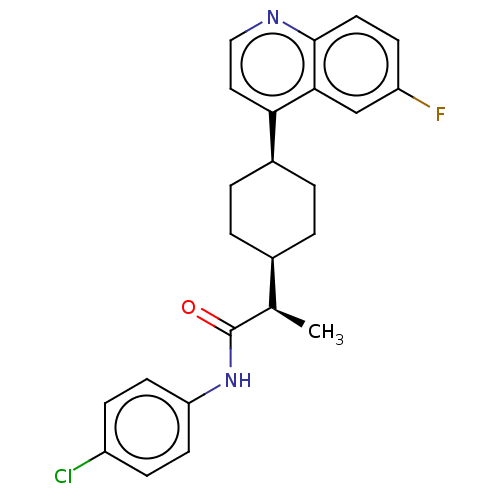
(CHEMBL4161733)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:18.21,1.0,wD:4.7,(66.16,-9.22,;66.17,-7.68,;66.16,-6.15,;67.5,-5.38,;68.84,-6.15,;68.83,-7.69,;67.5,-8.46,;70.18,-5.39,;71.5,-6.16,;72.84,-5.4,;72.84,-3.85,;71.5,-3.08,;71.5,-1.55,;70.17,-.79,;68.84,-1.56,;67.5,-.8,;68.85,-3.09,;70.18,-3.85,;64.83,-8.46,;64.84,-10,;63.5,-7.69,;63.49,-6.14,;62.16,-8.46,;60.83,-7.69,;59.49,-8.47,;58.15,-7.69,;58.16,-6.14,;56.82,-5.38,;59.49,-5.38,;60.83,-6.14,)| Show InChI InChI=1S/C24H24ClFN2O/c1-15(24(29)28-20-9-6-18(25)7-10-20)16-2-4-17(5-3-16)21-12-13-27-23-11-8-19(26)14-22(21)23/h6-17H,2-5H2,1H3,(H,28,29)/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50285416

(CHEMBL4161733)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:18.21,1.0,wD:4.7,(66.16,-9.22,;66.17,-7.68,;66.16,-6.15,;67.5,-5.38,;68.84,-6.15,;68.83,-7.69,;67.5,-8.46,;70.18,-5.39,;71.5,-6.16,;72.84,-5.4,;72.84,-3.85,;71.5,-3.08,;71.5,-1.55,;70.17,-.79,;68.84,-1.56,;67.5,-.8,;68.85,-3.09,;70.18,-3.85,;64.83,-8.46,;64.84,-10,;63.5,-7.69,;63.49,-6.14,;62.16,-8.46,;60.83,-7.69,;59.49,-8.47,;58.15,-7.69,;58.16,-6.14,;56.82,-5.38,;59.49,-5.38,;60.83,-6.14,)| Show InChI InChI=1S/C24H24ClFN2O/c1-15(24(29)28-20-9-6-18(25)7-10-20)16-2-4-17(5-3-16)21-12-13-27-23-11-8-19(26)14-22(21)23/h6-17H,2-5H2,1H3,(H,28,29)/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127744
BindingDB Entry DOI: 10.7270/Q29S1VP1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549540

(CHEMBL4745769)Show SMILES FC(F)(F)c1ccc2c(OCC22CCN(CC2)C(CC2CC2)C(=O)Nc2ccc(Cl)cc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549651
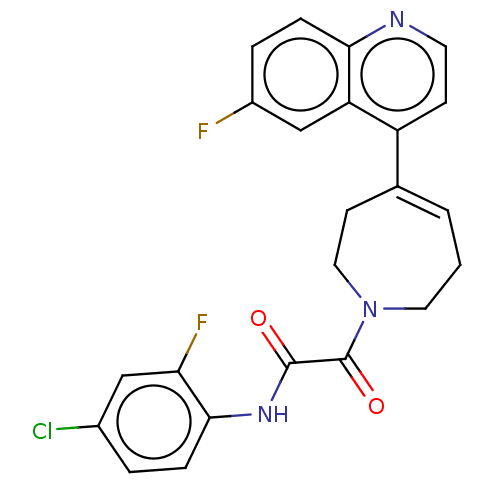
(CHEMBL4749799)Show SMILES Fc1ccc2nccc(C3=CCCN(CC3)C(=O)C(=O)Nc3ccc(Cl)cc3F)c2c1 |t:9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127744
BindingDB Entry DOI: 10.7270/Q29S1VP1 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549538

(CHEMBL4741630)Show SMILES [H][C@@]1(CC[C@]2(COc3cc(ccc23)C(F)(F)F)CC1)[C@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:4.4,19.22,1.0,(11.03,-49.44,;10.98,-50.89,;10.98,-52.43,;12.31,-53.2,;13.65,-52.42,;14.94,-51.58,;16.13,-52.55,;15.58,-53.98,;16.27,-55.34,;15.44,-56.62,;13.9,-56.54,;13.21,-55.17,;14.05,-53.9,;16.13,-57.99,;17.46,-57.21,;17.47,-58.75,;15.3,-59.28,;13.65,-50.88,;12.31,-50.11,;9.64,-50.12,;9.63,-48.58,;8.3,-50.9,;8.3,-52.44,;6.96,-50.13,;5.62,-50.91,;5.62,-52.46,;4.29,-53.24,;2.95,-52.46,;1.61,-53.24,;2.95,-50.91,;4.28,-50.14,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549546
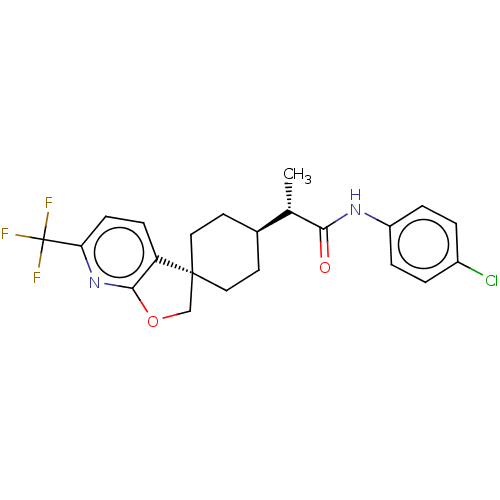
(CHEMBL4745907)Show SMILES [H][C@@]1(CC[C@@]2(COc3nc(ccc23)C(F)(F)F)CC1)[C@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:4.4,19.22,wD:1.0,(33.4,-3.4,;33.35,-4.96,;34.67,-4.18,;36.02,-4.95,;36.01,-6.49,;37.3,-5.66,;38.49,-6.62,;37.94,-8.06,;38.63,-9.41,;37.8,-10.69,;36.26,-10.61,;35.58,-9.24,;36.41,-7.97,;38.49,-12.05,;39.82,-11.28,;39.83,-12.81,;37.66,-13.35,;34.68,-7.27,;33.35,-6.5,;32.01,-4.2,;32,-2.66,;30.68,-4.97,;30.68,-6.51,;29.33,-4.21,;28,-4.98,;28,-6.53,;26.66,-7.31,;25.33,-6.53,;23.99,-7.31,;25.33,-4.99,;26.66,-4.22,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549640

(CHEMBL4753967)Show SMILES O[C@H]1CN(CC[C@@H]1c1ccnc2ccc(F)cc12)C(=O)C(=O)Nc1ccc(Cl)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127744
BindingDB Entry DOI: 10.7270/Q29S1VP1 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509644

(CHEMBL4538516)Show SMILES O\N=C(/Nc1ccc(F)c(c1)C(F)F)c1nonc1SCCNC(=O)CC#N Show InChI InChI=1S/C15H13F3N6O3S/c16-10-2-1-8(7-9(10)13(17)18)21-14(22-26)12-15(24-27-23-12)28-6-5-20-11(25)3-4-19/h1-2,7,13,26H,3,5-6H2,(H,20,25)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549548
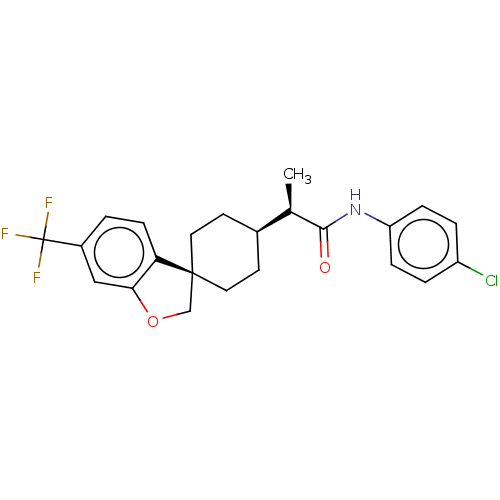
(CHEMBL4758026)Show SMILES [H][C@@]1(CC[C@]2(COc3cc(ccc23)C(F)(F)F)CC1)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:4.4,1.0,wD:19.22,(30.51,-48.63,;30.52,-50.25,;30.52,-51.79,;31.84,-52.56,;33.18,-51.78,;34.47,-50.95,;35.66,-51.91,;35.11,-53.34,;35.79,-54.7,;34.96,-55.98,;33.43,-55.9,;32.74,-54.53,;33.58,-53.26,;35.66,-57.34,;36.99,-56.57,;37,-58.1,;34.83,-58.64,;33.18,-50.24,;31.84,-49.47,;29.18,-49.48,;29.17,-47.95,;27.84,-50.26,;27.84,-51.8,;26.5,-49.5,;25.17,-50.27,;25.17,-51.82,;23.83,-52.6,;22.49,-51.82,;21.15,-52.6,;22.49,-50.27,;23.83,-49.51,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549662

(CHEMBL4740188)Show SMILES Fc1ccc2nccc(C3CCN(CC3)C(=O)C(=O)Nc3ccc(Cl)cc3F)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127744
BindingDB Entry DOI: 10.7270/Q29S1VP1 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549537

(CHEMBL4761104)Show SMILES [H][C@@]1(CC[C@@]2(COc3cc(ccc23)C(F)(F)F)CC1)[C@H](CC)C(=O)Nc1ccc(Cl)cn1 |r,wU:4.4,19.22,wD:1.0,(11.99,-3.45,;11.89,-5.07,;13.22,-4.29,;14.57,-5.06,;14.56,-6.6,;15.85,-5.77,;17.05,-6.74,;16.49,-8.17,;17.18,-9.53,;16.35,-10.81,;14.81,-10.73,;14.12,-9.36,;14.96,-8.08,;17.05,-12.18,;18.38,-11.4,;18.39,-12.94,;16.21,-13.48,;13.22,-7.38,;11.89,-6.61,;10.55,-4.3,;10.54,-2.76,;11.87,-1.99,;9.21,-5.08,;9.21,-6.62,;7.86,-4.31,;6.53,-5.09,;5.18,-4.32,;3.84,-5.09,;3.84,-6.65,;2.5,-7.42,;5.19,-7.42,;6.53,-6.65,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509629

(CHEMBL4436560)Show SMILES CS(=O)(=O)CCC(=O)NCCSc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C15H17BrFN5O5S2/c1-29(25,26)7-4-12(23)18-5-6-28-15-13(21-27-22-15)14(20-24)19-9-2-3-11(17)10(16)8-9/h2-3,8,24H,4-7H2,1H3,(H,18,23)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50549535

(CHEMBL4763375)Show SMILES [H][C@@]1(CC[C@@]2(COc3cc(ccc23)C(F)(F)F)CC1)[C@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:4.4,19.22,wD:1.0,(49.52,-49.21,;49.47,-50.71,;50.8,-49.93,;52.14,-50.7,;52.14,-52.25,;53.43,-51.41,;54.62,-52.38,;54.07,-53.81,;54.76,-55.17,;53.93,-56.45,;52.39,-56.37,;51.7,-55,;52.54,-53.72,;54.62,-57.81,;55.95,-57.04,;55.96,-58.58,;53.79,-59.11,;50.8,-53.03,;49.47,-52.25,;48.13,-49.95,;48.12,-48.41,;46.79,-50.72,;46.79,-52.27,;45.45,-49.96,;44.12,-50.73,;44.12,-52.29,;42.78,-53.07,;41.44,-52.29,;40.1,-53.06,;41.44,-50.74,;42.77,-49.97,)| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse IDO1 expressed in HEK cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549545
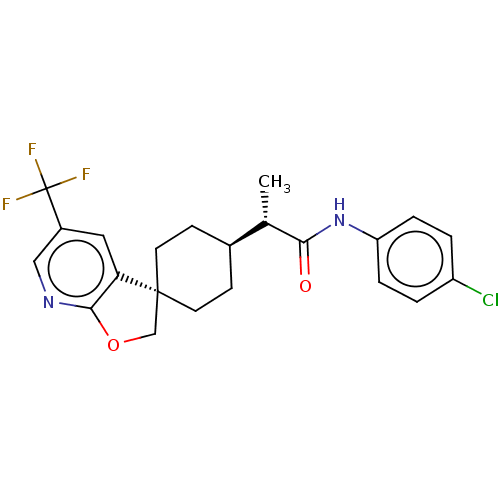
(CHEMBL4743724)Show SMILES [H][C@@]1(CC[C@@]2(COc3ncc(cc23)C(F)(F)F)CC1)[C@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:4.4,19.22,wD:1.0,(57.96,-4.25,;57.91,-5.87,;59.24,-5.09,;60.58,-5.86,;60.58,-7.4,;61.86,-6.56,;63.05,-7.53,;62.5,-8.96,;63.19,-10.32,;62.36,-11.6,;60.83,-11.51,;60.14,-10.15,;60.98,-8.87,;59.98,-12.8,;61.31,-13.56,;59.98,-14.33,;58.45,-12.72,;59.24,-8.18,;57.91,-7.41,;56.57,-5.1,;56.57,-3.57,;55.24,-5.88,;55.24,-7.42,;53.9,-5.11,;52.56,-5.89,;52.56,-7.44,;51.23,-8.22,;49.89,-7.44,;48.55,-8.22,;49.89,-5.89,;51.22,-5.13,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549650

(CHEMBL4764848)Show SMILES Fc1ccc2nccc(C3CCCN(CC3)C(=O)C(=O)Nc3ccc(Cl)cc3F)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127744
BindingDB Entry DOI: 10.7270/Q29S1VP1 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509636

(CHEMBL4441030)Show SMILES NS(=O)(=O)CC(=O)NCCSc1nonc1\C(Nc1cccc(Br)c1)=N\O Show InChI InChI=1S/C13H15BrN6O5S2/c14-8-2-1-3-9(6-8)17-12(18-22)11-13(20-25-19-11)26-5-4-16-10(21)7-27(15,23)24/h1-3,6,22H,4-5,7H2,(H,16,21)(H,17,18)(H2,15,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549544
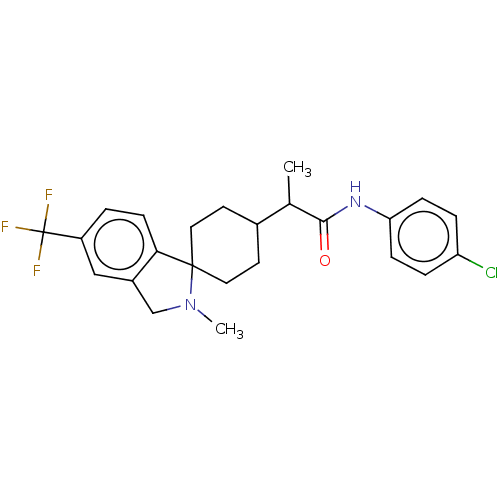
(CHEMBL4764360)Show SMILES CC(C1CCC2(CC1)N(C)Cc1cc(ccc21)C(F)(F)F)C(=O)Nc1ccc(Cl)cc1 |(10.52,-19.22,;10.52,-20.76,;11.87,-21.52,;11.87,-23.06,;13.19,-23.84,;14.53,-23.06,;14.53,-21.51,;13.19,-20.74,;15.82,-22.22,;15.9,-20.69,;17.01,-23.19,;16.46,-24.62,;17.15,-25.98,;16.32,-27.26,;14.78,-27.18,;14.09,-25.81,;14.93,-24.53,;17.01,-28.62,;18.34,-27.85,;18.35,-29.38,;16.18,-29.92,;9.19,-21.53,;9.19,-23.08,;7.84,-20.77,;6.51,-21.54,;6.51,-23.1,;5.17,-23.88,;3.83,-23.1,;2.49,-23.87,;3.83,-21.55,;5.17,-20.78,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509633

(CHEMBL4521926)Show SMILES CC(C)(O)CC(=O)NCCSc1nonc1\C(Nc1ccc(F)c(c1)C(F)F)=N\O Show InChI InChI=1S/C17H20F3N5O4S/c1-17(2,27)8-12(26)21-5-6-30-16-13(24-29-25-16)15(23-28)22-9-3-4-11(18)10(7-9)14(19)20/h3-4,7,14,27-28H,5-6,8H2,1-2H3,(H,21,26)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549648

(CHEMBL4777262)Show SMILES Fc1ccc2nccc(C3CCN(CC3(F)F)C(=O)C(=O)Nc3ccc(Cl)cc3)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127744
BindingDB Entry DOI: 10.7270/Q29S1VP1 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509638
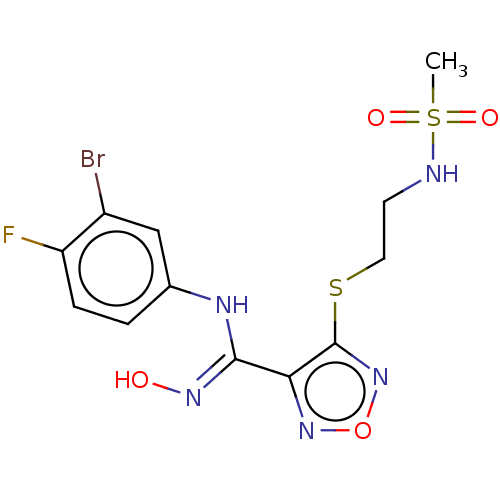
(CHEMBL4533342)Show SMILES CS(=O)(=O)NCCSc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C12H13BrFN5O4S2/c1-25(21,22)15-4-5-24-12-10(18-23-19-12)11(17-20)16-7-2-3-9(14)8(13)6-7/h2-3,6,15,20H,4-5H2,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509623
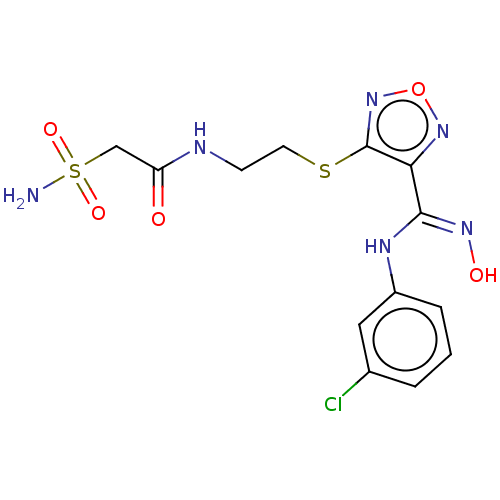
(CHEMBL4585089)Show SMILES NS(=O)(=O)CC(=O)NCCSc1nonc1\C(Nc1cccc(Cl)c1)=N\O Show InChI InChI=1S/C13H15ClN6O5S2/c14-8-2-1-3-9(6-8)17-12(18-22)11-13(20-25-19-11)26-5-4-16-10(21)7-27(15,23)24/h1-3,6,22H,4-5,7H2,(H,16,21)(H,17,18)(H2,15,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549550
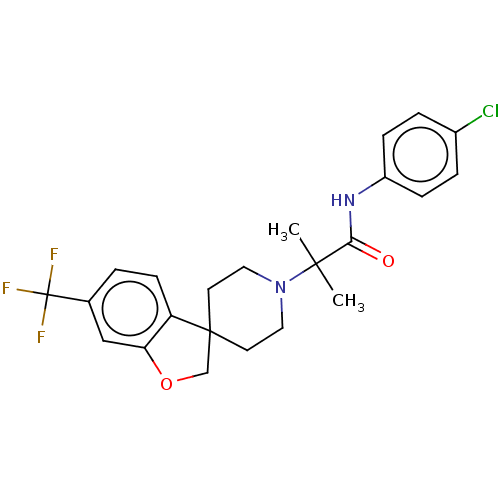
(CHEMBL4761629)Show SMILES CC(C)(N1CCC2(COc3cc(ccc23)C(F)(F)F)CC1)C(=O)Nc1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM430022

(US10538497, Example 212)Show SMILES NS(=O)(=O)NCCOc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H12BrFN6O5S/c12-7-5-6(1-2-8(7)13)16-10(17-20)9-11(19-24-18-9)23-4-3-15-25(14,21)22/h1-2,5,15,20H,3-4H2,(H,16,17)(H2,14,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509637
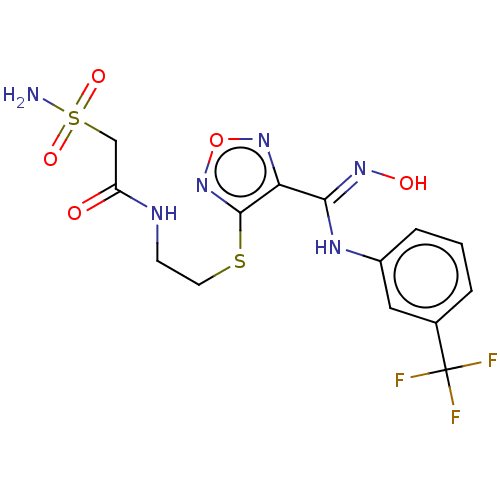
(CHEMBL4449948)Show SMILES NS(=O)(=O)CC(=O)NCCSc1nonc1\C(Nc1cccc(c1)C(F)(F)F)=N\O Show InChI InChI=1S/C14H15F3N6O5S2/c15-14(16,17)8-2-1-3-9(6-8)20-12(21-25)11-13(23-28-22-11)29-5-4-19-10(24)7-30(18,26)27/h1-3,6,25H,4-5,7H2,(H,19,24)(H,20,21)(H2,18,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549536
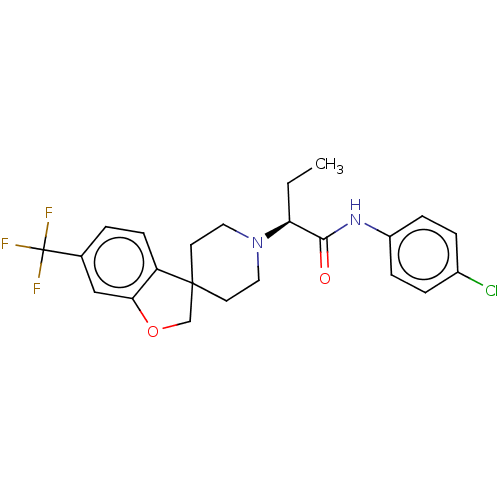
(CHEMBL4751227)Show SMILES CC[C@H](N1CCC2(COc3cc(ccc23)C(F)(F)F)CC1)C(=O)Nc1ccc(Cl)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509628

(CHEMBL4434929)Show SMILES C[C@H](CSc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O)NC(=O)CS(N)(=O)=O |r| Show InChI InChI=1S/C14H16BrFN6O5S2/c1-7(18-11(23)6-29(17,25)26)5-28-14-12(21-27-22-14)13(20-24)19-8-2-3-10(16)9(15)4-8/h2-4,7,24H,5-6H2,1H3,(H,18,23)(H,19,20)(H2,17,25,26)/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509630

(CHEMBL4448442)Show SMILES NS(=O)(=O)CC(=O)NCCSc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C13H14BrFN6O5S2/c14-8-5-7(1-2-9(8)15)18-12(19-23)11-13(21-26-20-11)27-4-3-17-10(22)6-28(16,24)25/h1-2,5,23H,3-4,6H2,(H,17,22)(H,18,19)(H2,16,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549539
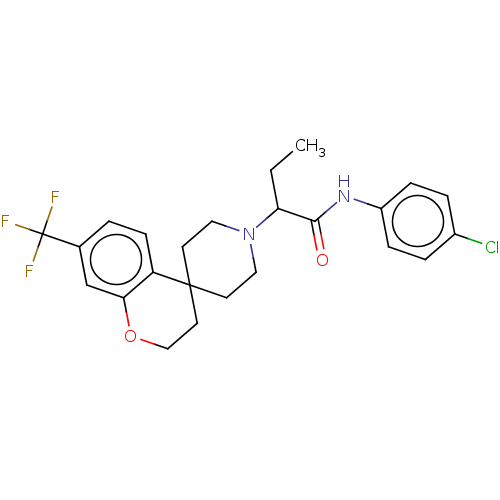
(CHEMBL4743826)Show SMILES CCC(N1CCC2(CCOc3cc(ccc23)C(F)(F)F)CC1)C(=O)Nc1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509634

(CHEMBL4437616)Show SMILES O\N=C(/Nc1ccc(F)c(c1)C(F)F)c1nonc1SCCNC(=O)C1CS(=O)(=O)C1 Show InChI InChI=1S/C16H16F3N5O5S2/c17-11-2-1-9(5-10(11)13(18)19)21-14(22-26)12-16(24-29-23-12)30-4-3-20-15(25)8-6-31(27,28)7-8/h1-2,5,8,13,26H,3-4,6-7H2,(H,20,25)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509641
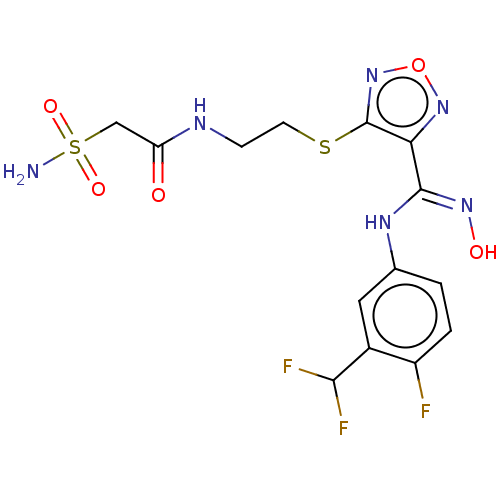
(CHEMBL4455438)Show SMILES NS(=O)(=O)CC(=O)NCCSc1nonc1\C(Nc1ccc(F)c(c1)C(F)F)=N\O Show InChI InChI=1S/C14H15F3N6O5S2/c15-9-2-1-7(5-8(9)12(16)17)20-13(21-25)11-14(23-28-22-11)29-4-3-19-10(24)6-30(18,26)27/h1-2,5,12,25H,3-4,6H2,(H,19,24)(H,20,21)(H2,18,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509639

(CHEMBL4439896)Show SMILES NS(=O)(=O)CC(=O)NCCSc1nonc1\C(Nc1ccc(F)c(c1)C(F)(F)F)=N\O Show InChI InChI=1S/C14H14F4N6O5S2/c15-9-2-1-7(5-8(9)14(16,17)18)21-12(22-26)11-13(24-29-23-11)30-4-3-20-10(25)6-31(19,27)28/h1-2,5,26H,3-4,6H2,(H,20,25)(H,21,22)(H2,19,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235921

(CHEMBL584991)Show InChI InChI=1S/C9H7ClFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127744
BindingDB Entry DOI: 10.7270/Q29S1VP1 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509632
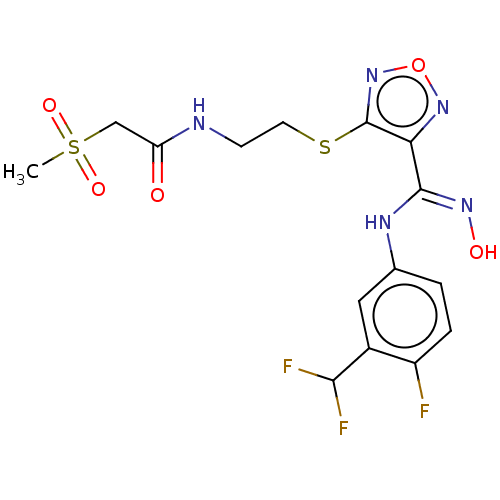
(CHEMBL4441246)Show SMILES CS(=O)(=O)CC(=O)NCCSc1nonc1\C(Nc1ccc(F)c(c1)C(F)F)=N\O Show InChI InChI=1S/C15H16F3N5O5S2/c1-30(26,27)7-11(24)19-4-5-29-15-12(22-28-23-15)14(21-25)20-8-2-3-10(16)9(6-8)13(17)18/h2-3,6,13,25H,4-5,7H2,1H3,(H,19,24)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509643

(CHEMBL4578545)Show SMILES CS(=N)(=O)CC(=O)NCCSc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C14H16BrFN6O4S2/c1-28(17,25)7-11(23)18-4-5-27-14-12(21-26-22-14)13(20-24)19-8-2-3-10(16)9(15)6-8/h2-3,6,17,24H,4-5,7H2,1H3,(H,18,23)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235921

(CHEMBL584991)Show InChI InChI=1S/C9H7ClFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549543

(CHEMBL4746699)Show SMILES Fc1ccc2nccc(C3CCN(CC3)C(=O)C(=O)Nc3ccc(Cl)cc3)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549543

(CHEMBL4746699)Show SMILES Fc1ccc2nccc(C3CCN(CC3)C(=O)C(=O)Nc3ccc(Cl)cc3)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127744
BindingDB Entry DOI: 10.7270/Q29S1VP1 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509625

(CHEMBL4573030)Show InChI InChI=1S/C10H8BrFN4O2S/c1-19-10-8(15-18-16-10)9(14-17)13-5-2-3-7(12)6(11)4-5/h2-4,17H,1H3,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509624

(CHEMBL4533432)Show SMILES CC1(CCN1)C(=O)NCCSc1nonc1\C(Nc1ccc(F)c(c1)C(F)F)=N\O Show InChI InChI=1S/C17H19F3N6O3S/c1-17(4-5-22-17)16(27)21-6-7-30-15-12(25-29-26-15)14(24-28)23-9-2-3-11(18)10(8-9)13(19)20/h2-3,8,13,22,28H,4-7H2,1H3,(H,21,27)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509627
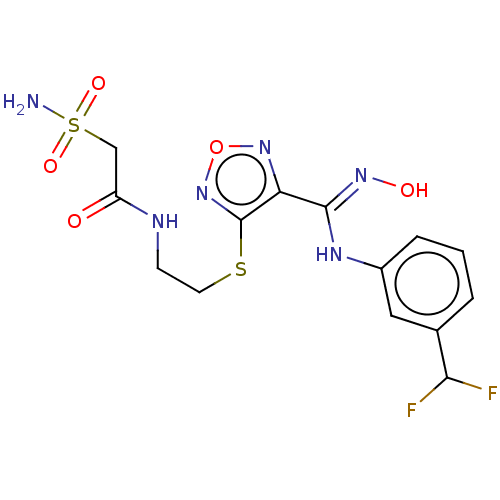
(CHEMBL4475499)Show SMILES NS(=O)(=O)CC(=O)NCCSc1nonc1\C(Nc1cccc(c1)C(F)F)=N\O Show InChI InChI=1S/C14H16F2N6O5S2/c15-12(16)8-2-1-3-9(6-8)19-13(20-24)11-14(22-27-21-11)28-5-4-18-10(23)7-29(17,25)26/h1-3,6,12,24H,4-5,7H2,(H,18,23)(H,19,20)(H2,17,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509631

(CHEMBL4451194)Show SMILES C[C@@H](CSc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O)NC(=O)CS(N)(=O)=O |r| Show InChI InChI=1S/C14H16BrFN6O5S2/c1-7(18-11(23)6-29(17,25)26)5-28-14-12(21-27-22-14)13(20-24)19-8-2-3-10(16)9(15)4-8/h2-4,7,24H,5-6H2,1H3,(H,18,23)(H,19,20)(H2,17,25,26)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549657
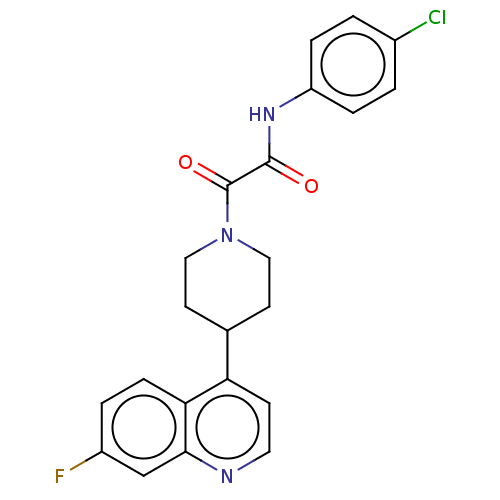
(CHEMBL4754544)Show SMILES Fc1ccc2c(ccnc2c1)C1CCN(CC1)C(=O)C(=O)Nc1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127744
BindingDB Entry DOI: 10.7270/Q29S1VP1 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549656

(CHEMBL4747072)Show SMILES Clc1ccc(NC(=O)C(=O)N2CCC(CC2)c2ccnc3ccc(Cl)cc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127744
BindingDB Entry DOI: 10.7270/Q29S1VP1 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509623

(CHEMBL4585089)Show SMILES NS(=O)(=O)CC(=O)NCCSc1nonc1\C(Nc1cccc(Cl)c1)=N\O Show InChI InChI=1S/C13H15ClN6O5S2/c14-8-2-1-3-9(6-8)17-12(18-22)11-13(20-25-19-11)26-5-4-16-10(21)7-27(15,23)24/h1-3,6,22H,4-5,7H2,(H,16,21)(H,17,18)(H2,15,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal GST-tagged IDO1 expressed in Escherichia coli Rosetta(DE3) pLysS using L-tryptophan as substrate measured after 1 hr b... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549659
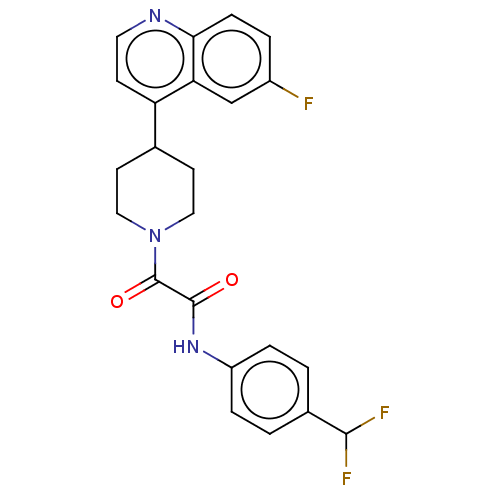
(CHEMBL4751316)Show SMILES FC(F)c1ccc(NC(=O)C(=O)N2CCC(CC2)c2ccnc3ccc(F)cc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127744
BindingDB Entry DOI: 10.7270/Q29S1VP1 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50509626
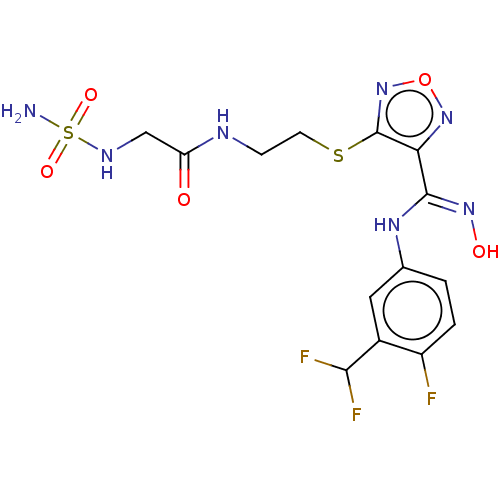
(CHEMBL4549976)Show SMILES NS(=O)(=O)NCC(=O)NCCSc1nonc1\C(Nc1ccc(F)c(c1)C(F)F)=N\O Show InChI InChI=1S/C14H16F3N7O5S2/c15-9-2-1-7(5-8(9)12(16)17)21-13(22-26)11-14(24-29-23-11)30-4-3-19-10(25)6-20-31(18,27)28/h1-2,5,12,20,26H,3-4,6H2,(H,19,25)(H,21,22)(H2,18,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in interferon-gamma-induced human SKOV3 cells assessed as N-formylkynurenine formation using L-tryptophan as substrate measured af... |
ACS Med Chem Lett 11: 179-187 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00572
BindingDB Entry DOI: 10.7270/Q2GX4FWT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50549541
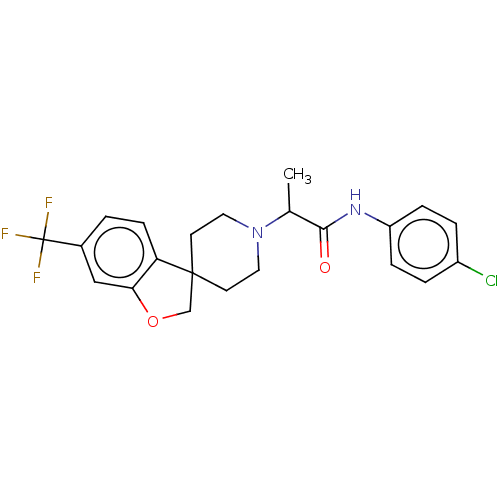
(CHEMBL4752478)Show SMILES CC(N1CCC2(COc3cc(ccc23)C(F)(F)F)CC1)C(=O)Nc1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human SKOV3 cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127738
BindingDB Entry DOI: 10.7270/Q270851B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data