 Found 34 hits with Last Name = 'iranshahi' and Initial = 'm'
Found 34 hits with Last Name = 'iranshahi' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50140172

(CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...)Show SMILES COc1cc(\C=C\C(=O)CC(=O)\C=C\c2ccc(O)c(OC)c2)ccc1O Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50500813
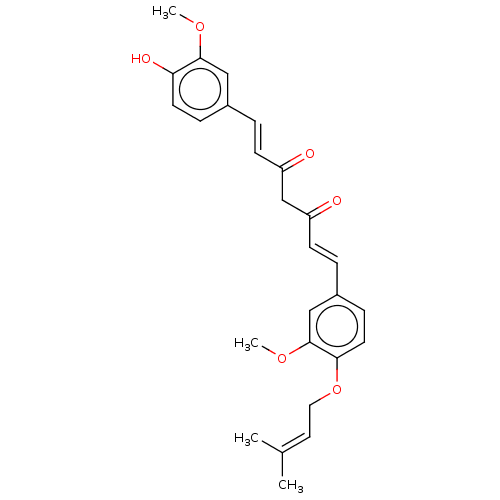
(CHEMBL1087690)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6]-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8] Show InChI InChI=1S/C26H28O6/c1-18(2)13-14-32-24-12-8-20(16-26(24)31-4)6-10-22(28)17-21(27)9-5-19-7-11-23(29)25(15-19)30-3/h5-13,15-16,29H,14,17H2,1-4H3/b9-5+,10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50500810
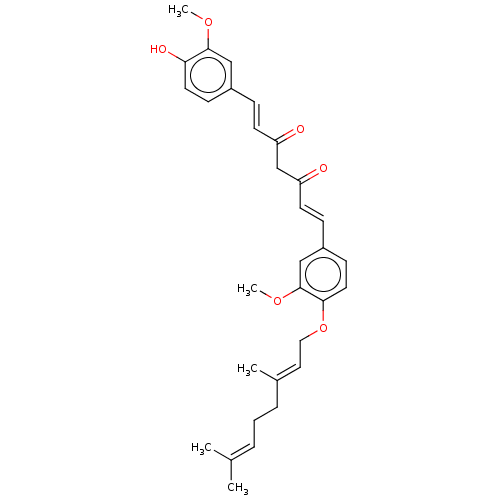
(CHEMBL3758791)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6]-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8] Show InChI InChI=1S/C31H36O6/c1-22(2)7-6-8-23(3)17-18-37-29-16-12-25(20-31(29)36-5)10-14-27(33)21-26(32)13-9-24-11-15-28(34)30(19-24)35-4/h7,9-17,19-20,34H,6,8,18,21H2,1-5H3/b13-9+,14-10+,23-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50391177

(CHEMBL2088235)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1ccc2oc(=O)ccc2c1 Show InChI InChI=1S/C24H30O3/c1-18(2)7-5-8-19(3)9-6-10-20(4)15-16-26-22-12-13-23-21(17-22)11-14-24(25)27-23/h7,9,11-15,17H,5-6,8,10,16H2,1-4H3/b19-9+,20-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 15-lipoxygenase by MBTH-DMAB method |
Eur J Med Chem 57: 134-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.006
BindingDB Entry DOI: 10.7270/Q20Z74CH |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50500809
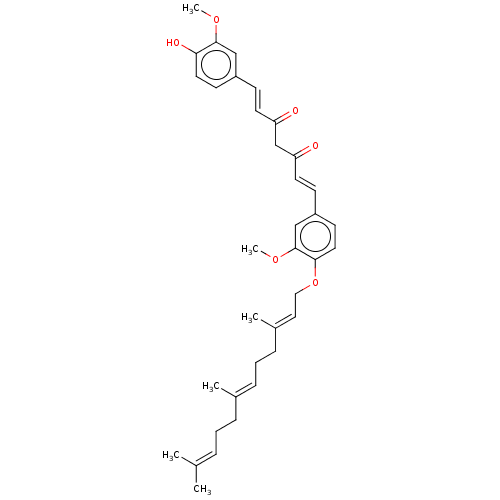
(CHEMBL3759529)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6]-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8] Show InChI InChI=1S/C36H44O6/c1-26(2)9-7-10-27(3)11-8-12-28(4)21-22-42-34-20-16-30(24-36(34)41-6)14-18-32(38)25-31(37)17-13-29-15-19-33(39)35(23-29)40-5/h9,11,13-21,23-24,39H,7-8,10,12,22,25H2,1-6H3/b17-13+,18-14+,27-11+,28-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50500817
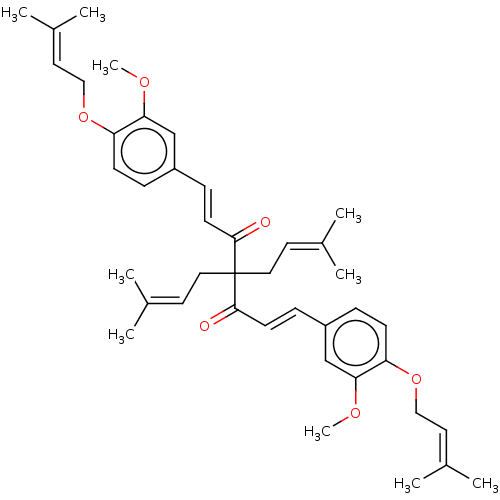
(CHEMBL3758432)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)C([#6]\[#6]=[#6](\[#6])-[#6])([#6]\[#6]=[#6](\[#6])-[#6])[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C41H52O6/c1-29(2)19-23-41(24-20-30(3)4,39(42)17-13-33-11-15-35(37(27-33)44-9)46-25-21-31(5)6)40(43)18-14-34-12-16-36(38(28-34)45-10)47-26-22-32(7)8/h11-22,27-28H,23-26H2,1-10H3/b17-13+,18-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50500816
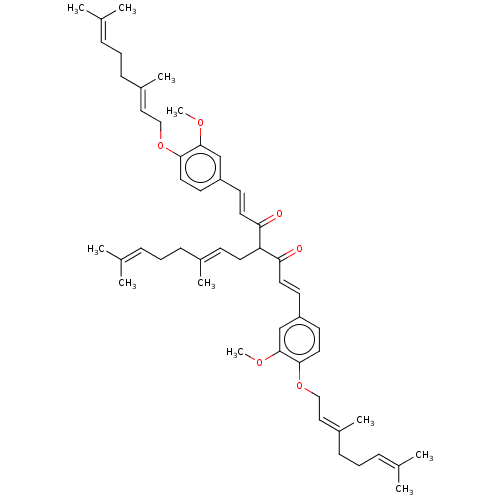
(CHEMBL3758656)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6](-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C51H68O6/c1-37(2)15-12-18-40(7)21-26-45(46(52)27-22-43-24-29-48(50(35-43)54-10)56-33-31-41(8)19-13-16-38(3)4)47(53)28-23-44-25-30-49(51(36-44)55-11)57-34-32-42(9)20-14-17-39(5)6/h15-17,21-25,27-32,35-36,45H,12-14,18-20,26,33-34H2,1-11H3/b27-22+,28-23+,40-21+,41-31+,42-32+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50500811
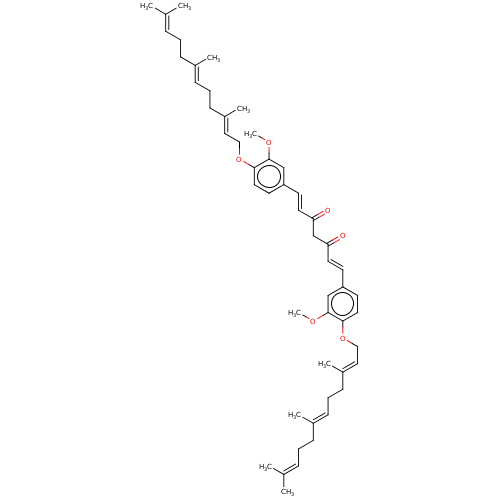
(CHEMBL3759749)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6]-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C51H68O6/c1-38(2)15-11-17-40(5)19-13-21-42(7)31-33-56-48-29-25-44(35-50(48)54-9)23-27-46(52)37-47(53)28-24-45-26-30-49(51(36-45)55-10)57-34-32-43(8)22-14-20-41(6)18-12-16-39(3)4/h15-16,19-20,23-32,35-36H,11-14,17-18,21-22,33-34,37H2,1-10H3/b27-23+,28-24+,40-19+,41-20+,42-31+,43-32+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50500814
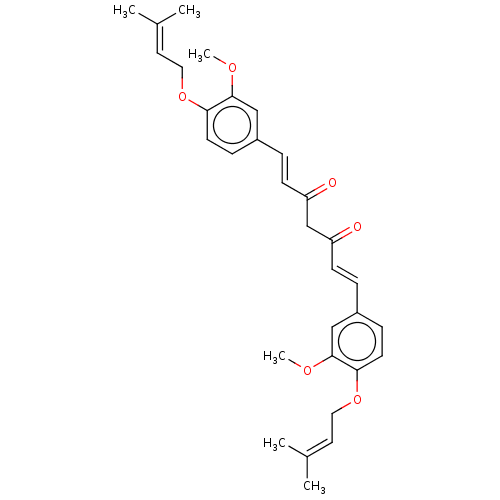
(CHEMBL1087807)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6]-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C31H36O6/c1-22(2)15-17-36-28-13-9-24(19-30(28)34-5)7-11-26(32)21-27(33)12-8-25-10-14-29(31(20-25)35-6)37-18-16-23(3)4/h7-16,19-20H,17-18,21H2,1-6H3/b11-7+,12-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50500812
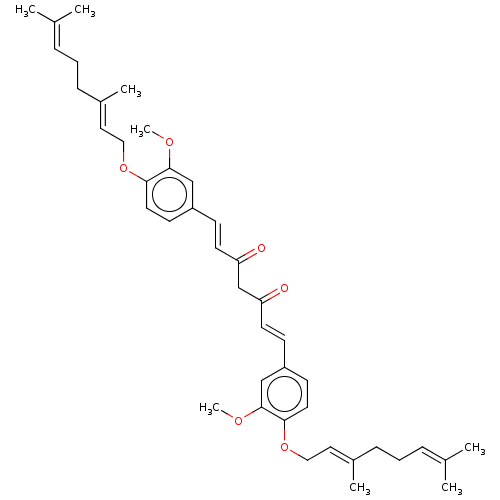
(CHEMBL3758253)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6]-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C41H52O6/c1-30(2)11-9-13-32(5)23-25-46-38-21-17-34(27-40(38)44-7)15-19-36(42)29-37(43)20-16-35-18-22-39(41(28-35)45-8)47-26-24-33(6)14-10-12-31(3)4/h11-12,15-24,27-28H,9-10,13-14,25-26,29H2,1-8H3/b19-15+,20-16+,32-23+,33-24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50500808
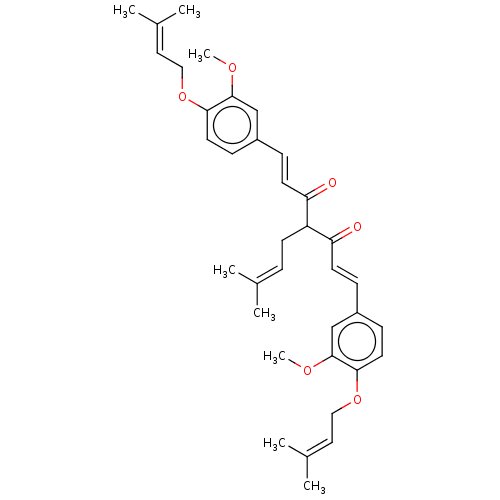
(CHEMBL3758528)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6](-[#6]\[#6]=[#6](\[#6])-[#6])-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C36H44O6/c1-25(2)9-14-30(31(37)15-10-28-12-17-33(35(23-28)39-7)41-21-19-26(3)4)32(38)16-11-29-13-18-34(36(24-29)40-8)42-22-20-27(5)6/h9-13,15-20,23-24,30H,14,21-22H2,1-8H3/b15-10+,16-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50500815
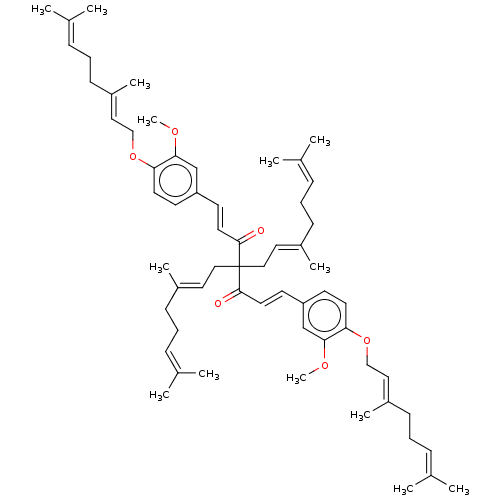
(CHEMBL3759699)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)C([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C61H84O6/c1-45(2)19-15-23-49(9)35-39-61(40-36-50(10)24-16-20-46(3)4,59(62)33-29-53-27-31-55(57(43-53)64-13)66-41-37-51(11)25-17-21-47(5)6)60(63)34-30-54-28-32-56(58(44-54)65-14)67-42-38-52(12)26-18-22-48(7)8/h19-22,27-38,43-44H,15-18,23-26,39-42H2,1-14H3/b33-29+,34-30+,49-35+,50-36+,51-37+,52-38+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50391176

(CHEMBL2088234)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1cccc2oc(=O)ccc12 Show InChI InChI=1S/C24H30O3/c1-18(2)8-5-9-19(3)10-6-11-20(4)16-17-26-22-12-7-13-23-21(22)14-15-24(25)27-23/h7-8,10,12-16H,5-6,9,11,17H2,1-4H3/b19-10+,20-16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 15-lipoxygenase by MBTH-DMAB method |
Eur J Med Chem 57: 134-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.006
BindingDB Entry DOI: 10.7270/Q20Z74CH |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50391172

(CHEMBL2088232)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1ccc2oc(=O)ccc2c1 Show InChI InChI=1S/C19H22O3/c1-14(2)5-4-6-15(3)11-12-21-17-8-9-18-16(13-17)7-10-19(20)22-18/h5,7-11,13H,4,6,12H2,1-3H3/b15-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 15-lipoxygenase by MBTH-DMAB method |
Eur J Med Chem 57: 134-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.006
BindingDB Entry DOI: 10.7270/Q20Z74CH |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50391171

(CHEMBL2088231)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1cccc2oc(=O)ccc12 Show InChI InChI=1S/C19H22O3/c1-14(2)6-4-7-15(3)12-13-21-17-8-5-9-18-16(17)10-11-19(20)22-18/h5-6,8-12H,4,7,13H2,1-3H3/b15-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 15-lipoxygenase by MBTH-DMAB method |
Eur J Med Chem 57: 134-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.006
BindingDB Entry DOI: 10.7270/Q20Z74CH |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50391178
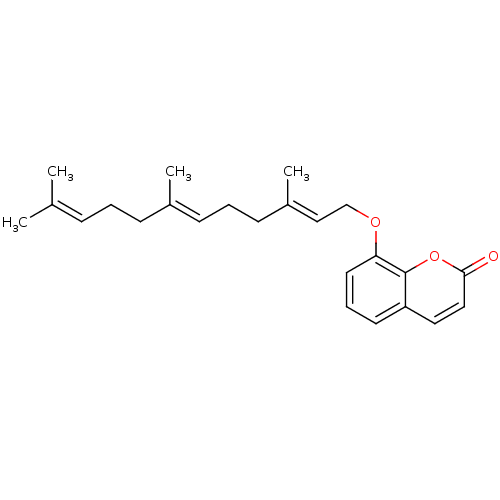
(CHEMBL2088236)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1cccc2ccc(=O)oc12 Show InChI InChI=1S/C24H30O3/c1-18(2)8-5-9-19(3)10-6-11-20(4)16-17-26-22-13-7-12-21-14-15-23(25)27-24(21)22/h7-8,10,12-16H,5-6,9,11,17H2,1-4H3/b19-10+,20-16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 15-lipoxygenase by MBTH-DMAB method |
Eur J Med Chem 57: 134-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.006
BindingDB Entry DOI: 10.7270/Q20Z74CH |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50143435
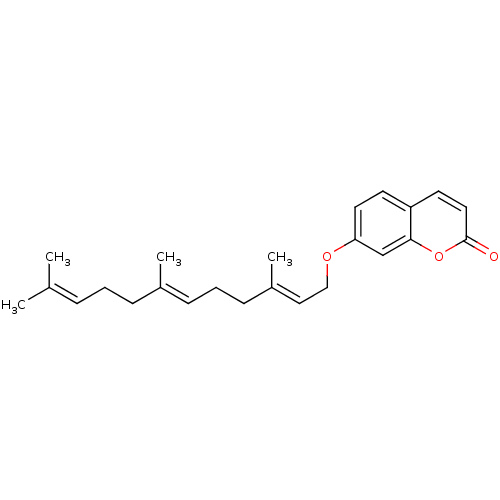
(7-((2E,6E)-3,7,11-Trimethyl-dodeca-2,6,10-trienylo...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1ccc2ccc(=O)oc2c1 Show InChI InChI=1S/C24H30O3/c1-18(2)7-5-8-19(3)9-6-10-20(4)15-16-26-22-13-11-21-12-14-24(25)27-23(21)17-22/h7,9,11-15,17H,5-6,8,10,16H2,1-4H3/b19-9+,20-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 15-lipoxygenase by MBTH-DMAB method |
Eur J Med Chem 57: 134-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.006
BindingDB Entry DOI: 10.7270/Q20Z74CH |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50361373
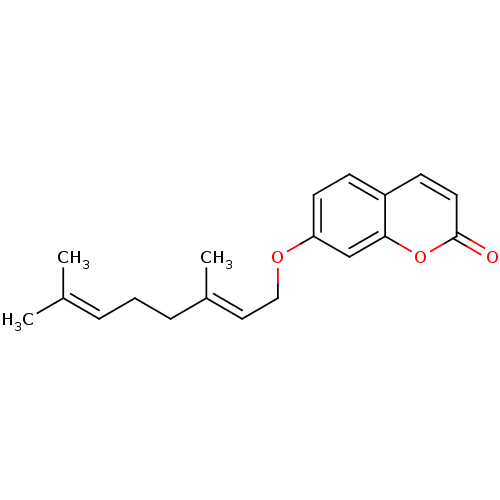
(AURAPTENE)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1ccc2ccc(=O)oc2c1 Show InChI InChI=1S/C19H22O3/c1-14(2)5-4-6-15(3)11-12-21-17-9-7-16-8-10-19(20)22-18(16)13-17/h5,7-11,13H,4,6,12H2,1-3H3/b15-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 15-lipoxygenase by MBTH-DMAB method |
Eur J Med Chem 57: 134-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.006
BindingDB Entry DOI: 10.7270/Q20Z74CH |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50391175
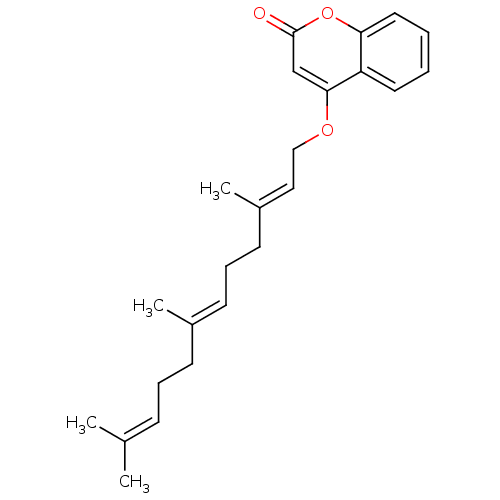
(CHEMBL177085)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1cc(=O)oc2ccccc12 Show InChI InChI=1S/C24H30O3/c1-18(2)9-7-10-19(3)11-8-12-20(4)15-16-26-23-17-24(25)27-22-14-6-5-13-21(22)23/h5-6,9,11,13-15,17H,7-8,10,12,16H2,1-4H3/b19-11+,20-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 15-lipoxygenase by MBTH-DMAB method |
Eur J Med Chem 57: 134-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.006
BindingDB Entry DOI: 10.7270/Q20Z74CH |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50391173

(CHEMBL2088233)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1cccc2ccc(=O)oc12 Show InChI InChI=1S/C19H22O3/c1-14(2)6-4-7-15(3)12-13-21-17-9-5-8-16-10-11-18(20)22-19(16)17/h5-6,8-12H,4,7,13H2,1-3H3/b15-12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 15-lipoxygenase by MBTH-DMAB method |
Eur J Med Chem 57: 134-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.006
BindingDB Entry DOI: 10.7270/Q20Z74CH |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50391170
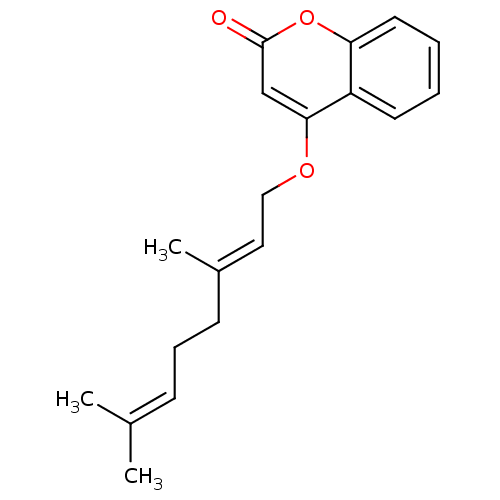
(CHEMBL2088230)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1cc(=O)oc2ccccc12 Show InChI InChI=1S/C19H22O3/c1-14(2)7-6-8-15(3)11-12-21-18-13-19(20)22-17-10-5-4-9-16(17)18/h4-5,7,9-11,13H,6,8,12H2,1-3H3/b15-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 15-lipoxygenase by MBTH-DMAB method |
Eur J Med Chem 57: 134-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.006
BindingDB Entry DOI: 10.7270/Q20Z74CH |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50391179

(CHEMBL2088237)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1ccccc1 Show InChI InChI=1S/C21H30O/c1-18(2)10-8-11-19(3)12-9-13-20(4)16-17-22-21-14-6-5-7-15-21/h5-7,10,12,14-16H,8-9,11,13,17H2,1-4H3/b19-12+,20-16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 15-lipoxygenase by MBTH-DMAB method |
Eur J Med Chem 57: 134-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.006
BindingDB Entry DOI: 10.7270/Q20Z74CH |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50391174

(CHEMBL1834532)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1cc2ccccc2oc1=O Show InChI InChI=1S/C24H30O3/c1-18(2)9-7-10-19(3)11-8-12-20(4)15-16-26-23-17-21-13-5-6-14-22(21)27-24(23)25/h5-6,9,11,13-15,17H,7-8,10,12,16H2,1-4H3/b19-11+,20-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 15-lipoxygenase by MBTH-DMAB method |
Eur J Med Chem 57: 134-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.006
BindingDB Entry DOI: 10.7270/Q20Z74CH |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50500809

(CHEMBL3759529)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6]-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8] Show InChI InChI=1S/C36H44O6/c1-26(2)9-7-10-27(3)11-8-12-28(4)21-22-42-34-20-16-30(24-36(34)41-6)14-18-32(38)25-31(37)17-13-29-15-19-33(39)35(23-29)40-5/h9,11,13-21,23-24,39H,7-8,10,12,22,25H2,1-6H3/b17-13+,18-14+,27-11+,28-21+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50391169

(CHEMBL2086412)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1cc2ccccc2oc1=O Show InChI InChI=1S/C19H22O3/c1-14(2)7-6-8-15(3)11-12-21-18-13-16-9-4-5-10-17(16)22-19(18)20/h4-5,7,9-11,13H,6,8,12H2,1-3H3/b15-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 15-lipoxygenase by MBTH-DMAB method |
Eur J Med Chem 57: 134-42 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.006
BindingDB Entry DOI: 10.7270/Q20Z74CH |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50500811

(CHEMBL3759749)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6]-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C51H68O6/c1-38(2)15-11-17-40(5)19-13-21-42(7)31-33-56-48-29-25-44(35-50(48)54-9)23-27-46(52)37-47(53)28-24-45-26-30-49(51(36-45)55-10)57-34-32-43(8)22-14-20-41(6)18-12-16-39(3)4/h15-16,19-20,23-32,35-36H,11-14,17-18,21-22,33-34,37H2,1-10H3/b27-23+,28-24+,40-19+,41-20+,42-31+,43-32+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50140172

(CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...)Show SMILES COc1cc(\C=C\C(=O)CC(=O)\C=C\c2ccc(O)c(OC)c2)ccc1O Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3/b7-3+,8-4+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50500812

(CHEMBL3758253)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6]-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C41H52O6/c1-30(2)11-9-13-32(5)23-25-46-38-21-17-34(27-40(38)44-7)15-19-36(42)29-37(43)20-16-35-18-22-39(41(28-35)45-8)47-26-24-33(6)14-10-12-31(3)4/h11-12,15-24,27-28H,9-10,13-14,25-26,29H2,1-8H3/b19-15+,20-16+,32-23+,33-24+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50500816

(CHEMBL3758656)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6](-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C51H68O6/c1-37(2)15-12-18-40(7)21-26-45(46(52)27-22-43-24-29-48(50(35-43)54-10)56-33-31-41(8)19-13-16-38(3)4)47(53)28-23-44-25-30-49(51(36-44)55-11)57-34-32-42(9)20-14-17-39(5)6/h15-17,21-25,27-32,35-36,45H,12-14,18-20,26,33-34H2,1-11H3/b27-22+,28-23+,40-21+,41-31+,42-32+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50500814

(CHEMBL1087807)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6]-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C31H36O6/c1-22(2)15-17-36-28-13-9-24(19-30(28)34-5)7-11-26(32)21-27(33)12-8-25-10-14-29(31(20-25)35-6)37-18-16-23(3)4/h7-16,19-20H,17-18,21H2,1-6H3/b11-7+,12-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50500810

(CHEMBL3758791)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6]-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8] Show InChI InChI=1S/C31H36O6/c1-22(2)7-6-8-23(3)17-18-37-29-16-12-25(20-31(29)36-5)10-14-27(33)21-26(32)13-9-24-11-15-28(34)30(19-24)35-4/h7,9-17,19-20,34H,6,8,18,21H2,1-5H3/b13-9+,14-10+,23-17+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50500815

(CHEMBL3759699)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)C([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C61H84O6/c1-45(2)19-15-23-49(9)35-39-61(40-36-50(10)24-16-20-46(3)4,59(62)33-29-53-27-31-55(57(43-53)64-13)66-41-37-51(11)25-17-21-47(5)6)60(63)34-30-54-28-32-56(58(44-54)65-14)67-42-38-52(12)26-18-22-48(7)8/h19-22,27-38,43-44H,15-18,23-26,39-42H2,1-14H3/b33-29+,34-30+,49-35+,50-36+,51-37+,52-38+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50500813

(CHEMBL1087690)Show SMILES [#6]-[#8]-c1cc(\[#6]=[#6]\[#6](=O)-[#6]-[#6](=O)\[#6]=[#6]\c2ccc(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8]-[#6])c2)ccc1-[#8] Show InChI InChI=1S/C26H28O6/c1-18(2)13-14-32-24-12-8-20(16-26(24)31-4)6-10-22(28)17-21(27)9-5-19-7-11-23(29)25(15-19)30-3/h5-13,15-16,29H,14,17H2,1-4H3/b9-5+,10-6+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.62E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data