

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524981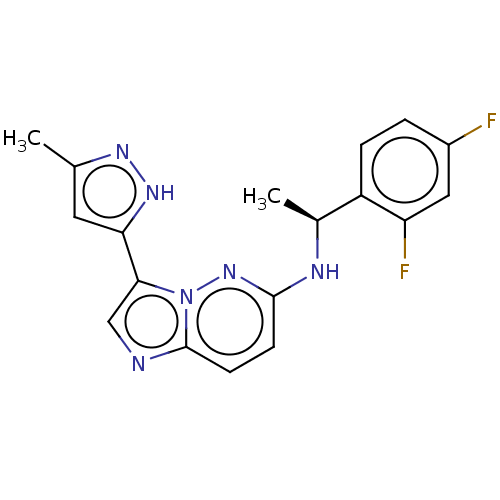 (CHEMBL4562879) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524985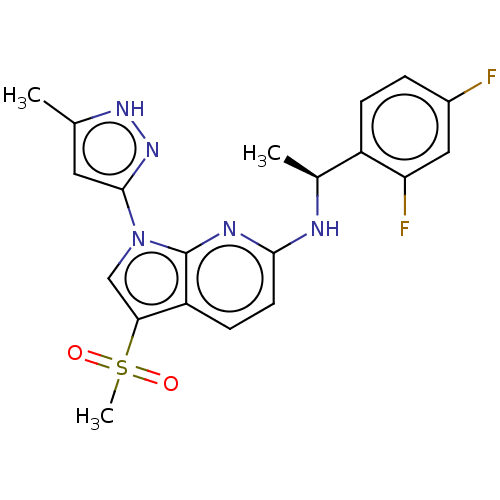 (CHEMBL4460367) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524987 (CHEMBL4516801) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50306682 ((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524983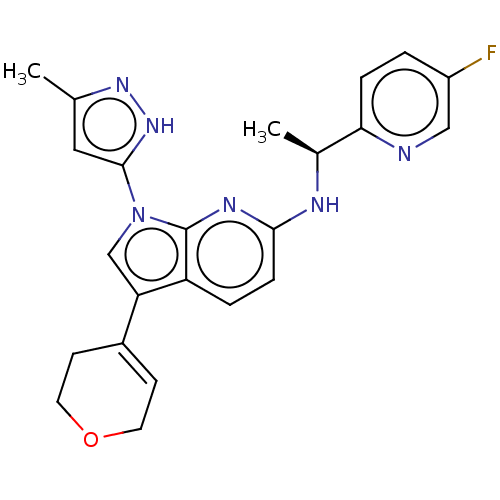 (CHEMBL4458269) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524981 (CHEMBL4562879) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524980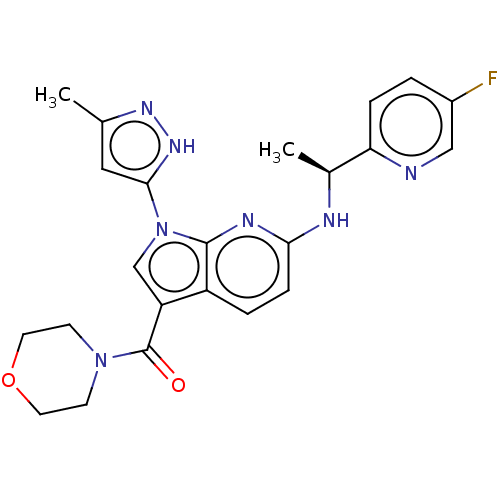 (CHEMBL4437605) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524983 (CHEMBL4458269) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524979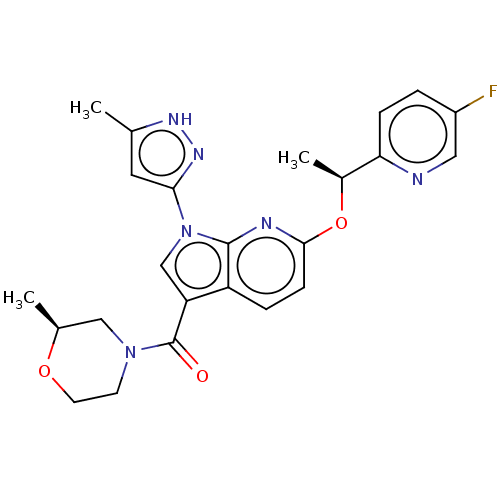 (CHEMBL4440381) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524984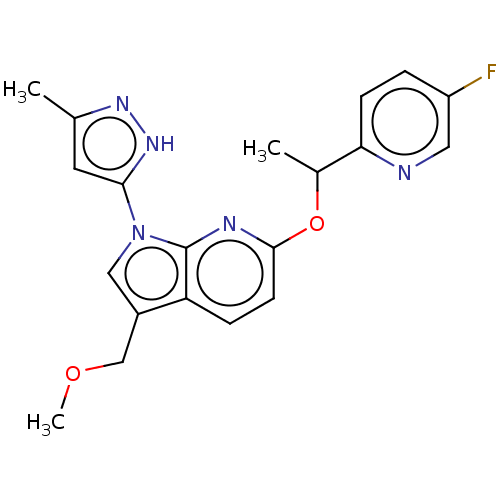 (CHEMBL4434659) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524984 (CHEMBL4434659) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524989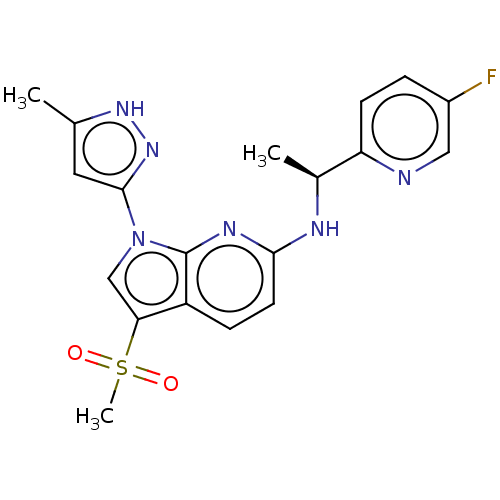 (CHEMBL4535072) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524978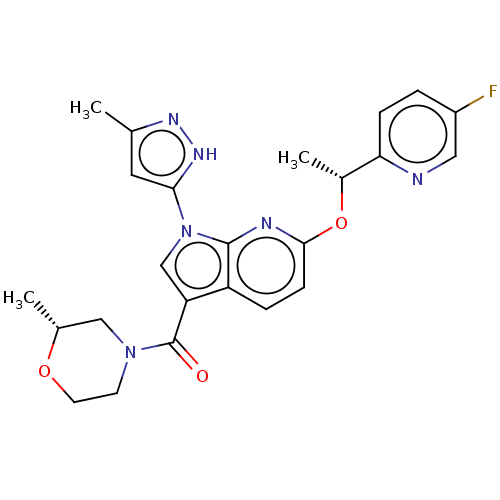 (CHEMBL4573505) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524981 (CHEMBL4562879) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524988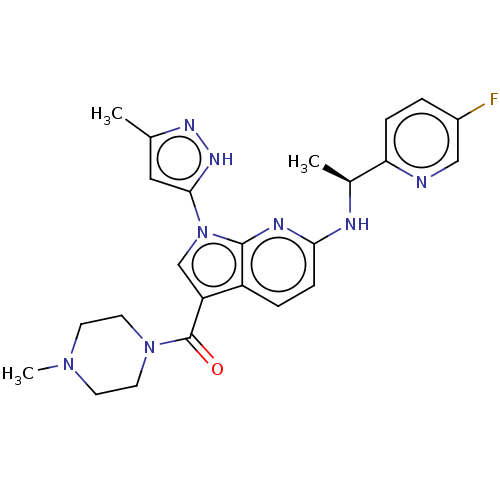 (CHEMBL4586773) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524975 (CHEMBL4476859) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524983 (CHEMBL4458269) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524977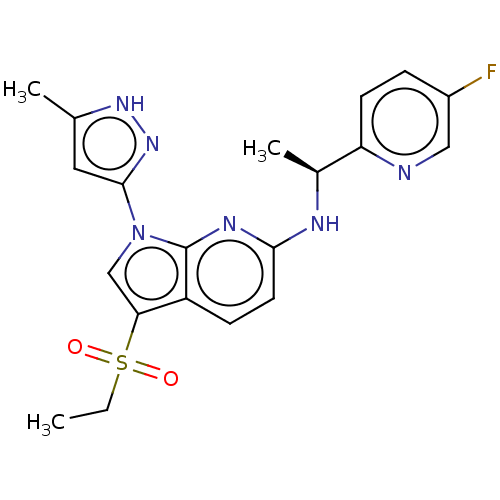 (CHEMBL4443254) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524984 (CHEMBL4434659) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524982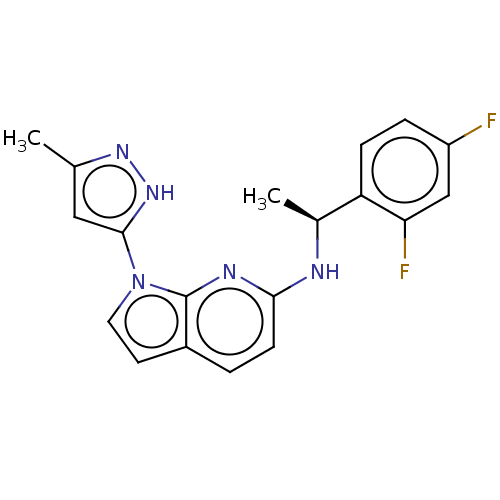 (CHEMBL4448434) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524989 (CHEMBL4535072) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524982 (CHEMBL4448434) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524985 (CHEMBL4460367) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524987 (CHEMBL4516801) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524976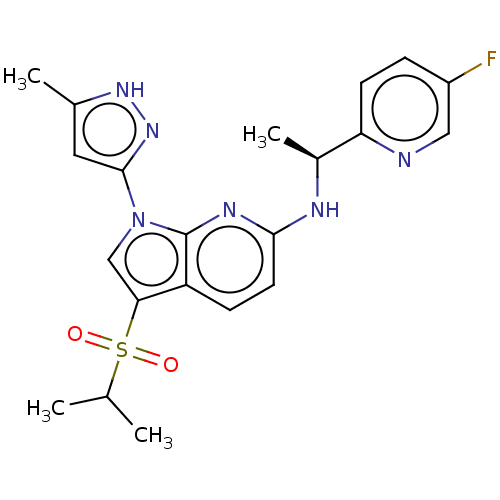 (CHEMBL4435574) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524986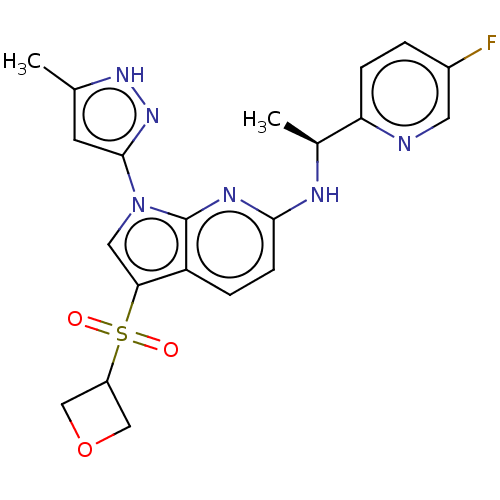 (CHEMBL4516124) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524980 (CHEMBL4437605) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50524979 (CHEMBL4440381) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human FAK kinase domain (411 to 686 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incubate... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524985 (CHEMBL4460367) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524979 (CHEMBL4440381) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50306682 ((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524977 (CHEMBL4443254) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524988 (CHEMBL4586773) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524978 (CHEMBL4573505) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524989 (CHEMBL4535072) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524986 (CHEMBL4516124) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524975 (CHEMBL4476859) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50242067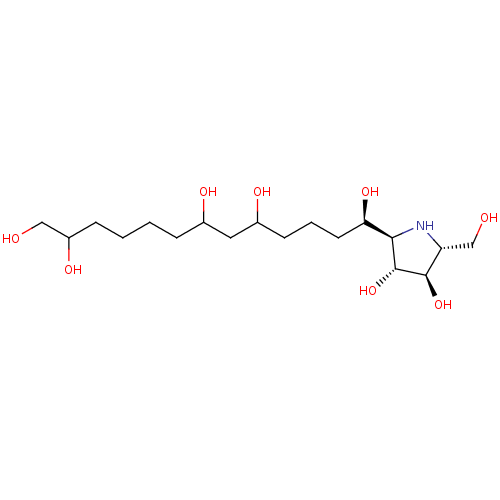 (CHEMBL469435 | alpha-1-C-(1,10,13-Trihydroxytridec...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase by spectrophotometry | J Nat Prod 67: 846-50 (2004) Article DOI: 10.1021/np0499721 BindingDB Entry DOI: 10.7270/Q2Q52PD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524977 (CHEMBL4443254) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524987 (CHEMBL4516801) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524982 (CHEMBL4448434) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524988 (CHEMBL4586773) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524976 (CHEMBL4435574) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524986 (CHEMBL4516124) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50524990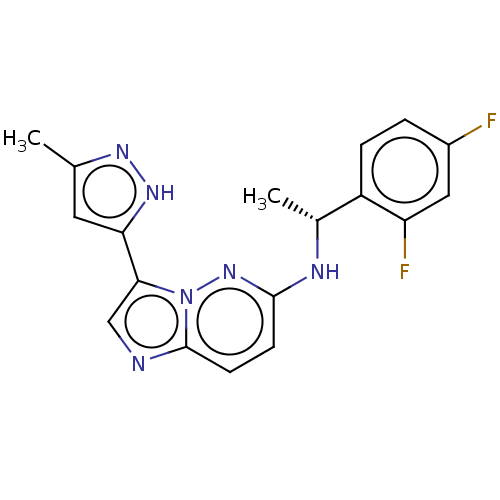 (CHEMBL4529040) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524980 (CHEMBL4437605) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524975 (CHEMBL4476859) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524984 (CHEMBL4434659) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524976 (CHEMBL4435574) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50524979 (CHEMBL4440381) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as... | J Med Chem 62: 4915-4935 (2019) Article DOI: 10.1021/acs.jmedchem.8b01630 BindingDB Entry DOI: 10.7270/Q2B56P57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |