 Found 394 hits with Last Name = 'matteo' and Initial = 'm'
Found 394 hits with Last Name = 'matteo' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM13211
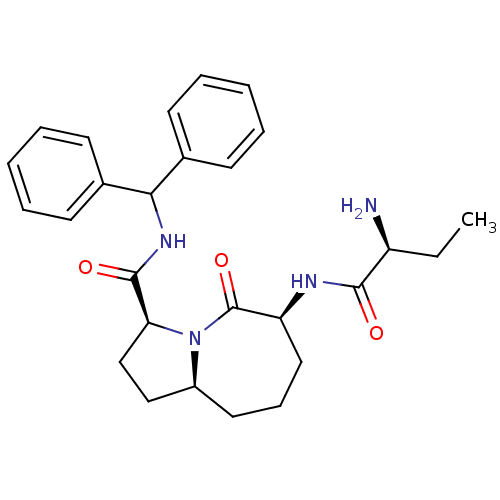
((3S,6S,9aS)-6-[(2S)-2-aminobutanamido]-N-(diphenyl...)Show SMILES CC[C@H](N)C(=O)N[C@H]1CCC[C@H]2CC[C@H](N2C1=O)C(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C27H34N4O3/c1-2-21(28)25(32)29-22-15-9-14-20-16-17-23(31(20)27(22)34)26(33)30-24(18-10-5-3-6-11-18)19-12-7-4-8-13-19/h3-8,10-13,20-24H,2,9,14-17,28H2,1H3,(H,29,32)(H,30,33)/t20-,21-,22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | -43.1 | 460 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Universita degli Studi di Milano
| Assay Description
Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... |
Bioorg Med Chem 17: 5834-56 (2009)
Article DOI: 10.1016/j.bmc.2009.07.009
BindingDB Entry DOI: 10.7270/Q28W3BNV |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM13212
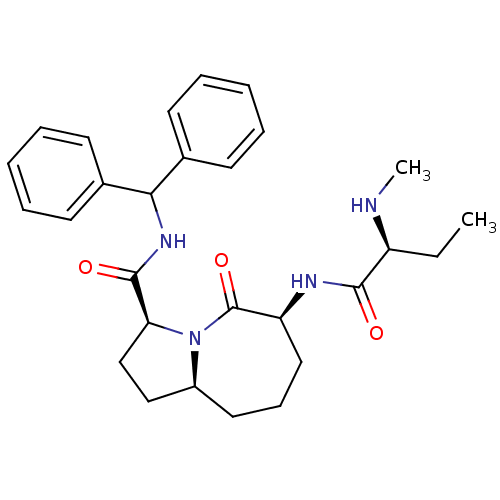
((3S,6S,9aS)-N-(diphenylmethyl)-6-[(2S)-2-(methylam...)Show SMILES CC[C@H](NC)C(=O)N[C@H]1CCC[C@H]2CC[C@H](N2C1=O)C(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C28H36N4O3/c1-3-22(29-2)26(33)30-23-16-10-15-21-17-18-24(32(21)28(23)35)27(34)31-25(19-11-6-4-7-12-19)20-13-8-5-9-14-20/h4-9,11-14,21-25,29H,3,10,15-18H2,1-2H3,(H,30,33)(H,31,34)/t21-,22-,23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 61 | -40.9 | 530 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Universita degli Studi di Milano
| Assay Description
Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... |
Bioorg Med Chem 17: 5834-56 (2009)
Article DOI: 10.1016/j.bmc.2009.07.009
BindingDB Entry DOI: 10.7270/Q28W3BNV |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50592391
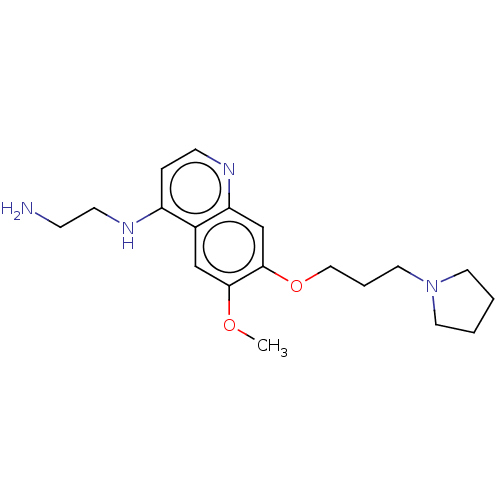
(CHEMBL5187766) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615001

(CHEMBL5267582)Show SMILES [H][C@@]12CCN(C[C@]1([H])[C@@]2(CN)c1nc(C)cs1)c1cnc2c(n[nH]c2n1)-c1ccc2nc(C)sc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50592384
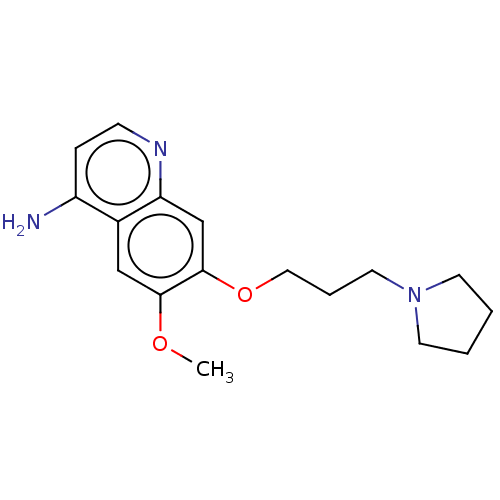
(CHEMBL5195846) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50446376
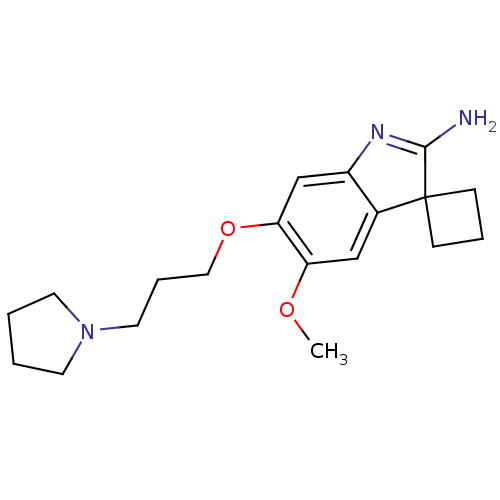
(CHEMBL3109630)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(N)C21CCC1 |t:19| Show InChI InChI=1S/C19H27N3O2/c1-23-16-12-14-15(21-18(20)19(14)6-4-7-19)13-17(16)24-11-5-10-22-8-2-3-9-22/h12-13H,2-11H2,1H3,(H2,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50614997
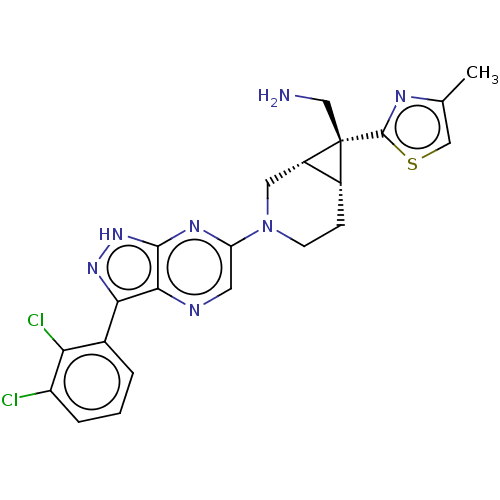
(CHEMBL5281311)Show SMILES [H][C@@]12CCN(C[C@]1([H])[C@]2(CN)c1nc(C)cs1)c1cnc2c(n[nH]c2n1)-c1cccc(Cl)c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615002

(CHEMBL5282278)Show SMILES [H][C@@]12CCN(C[C@]1([H])[C@@]2(CN)c1nc(C)cs1)c1cnc2c(n[nH]c2n1)-c1ccn2nccc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50546219
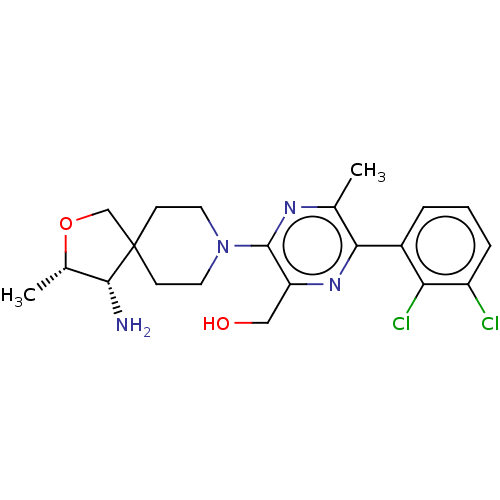
(CHEMBL4752026 | US11596633, Compound B | US1170239...)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2nc(C)c(nc2CO)-c2cccc(Cl)c2Cl)[C@@H]1N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615005
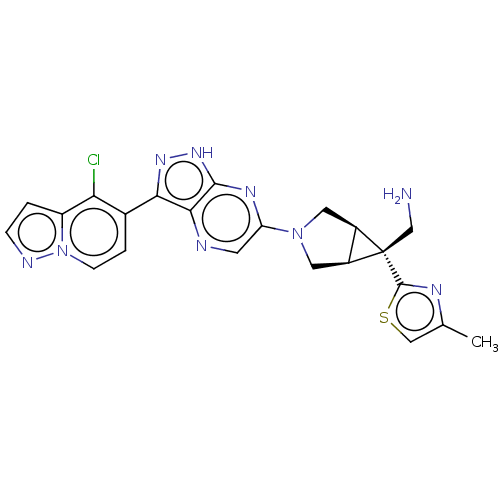
(CHEMBL5291336)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]2(CN)c1nc(C)cs1)c1cnc2c(n[nH]c2n1)-c1ccn2nccc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615009
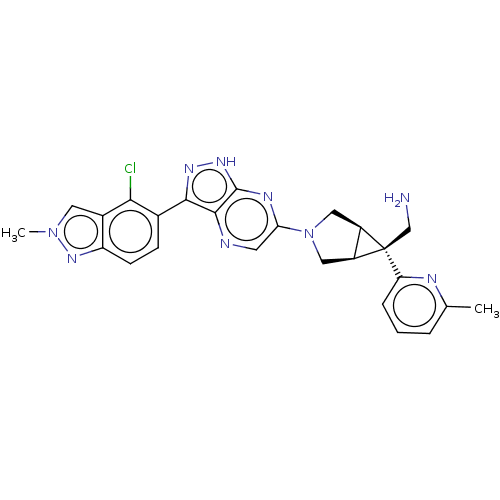
(CHEMBL5271919)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]2(CN)c1cccc(C)n1)c1cnc2c(n[nH]c2n1)-c1ccc2nn(C)cc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615013
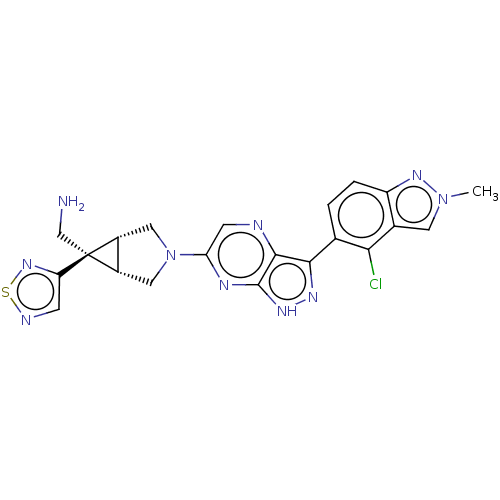
(CHEMBL5268577)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]2(CN)c1cnsn1)c1cnc2c(n[nH]c2n1)-c1ccc2nn(C)cc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615015
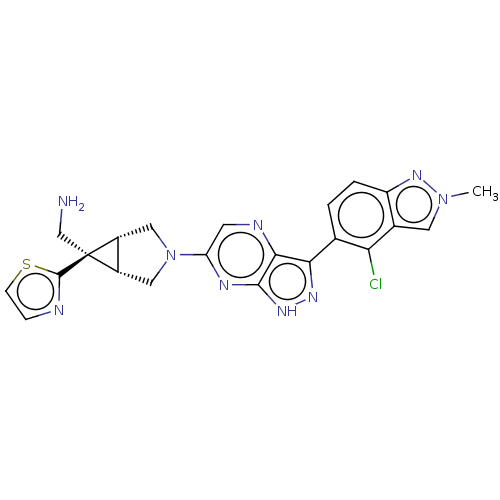
(CHEMBL5289856)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]2(CN)c1nccs1)c1cnc2c(n[nH]c2n1)-c1ccc2nn(C)cc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM13061

(4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...)Show InChI InChI=1S/C17H11N5/c18-9-13-1-5-15(6-2-13)17(22-12-20-11-21-22)16-7-3-14(10-19)4-8-16/h1-8,11-12,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50614995
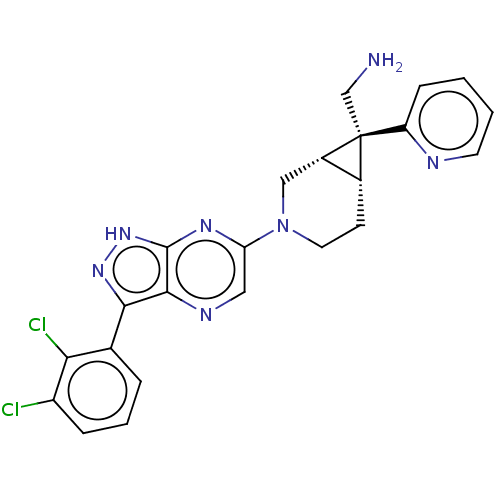
(CHEMBL5270277)Show SMILES [H][C@@]12CCN(C[C@]1([H])[C@@]2(CN)c1ccccn1)c1cnc2c(n[nH]c2n1)-c1cccc(Cl)c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615004
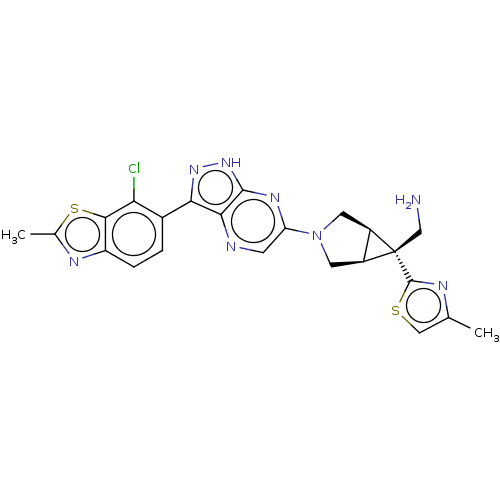
(CHEMBL5275370)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]2(CN)c1nc(C)cs1)c1cnc2c(n[nH]c2n1)-c1ccc2nc(C)sc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615006
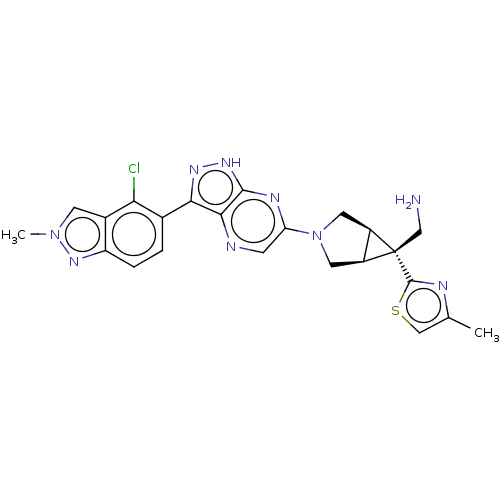
(CHEMBL5290261)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]2(CN)c1nc(C)cs1)c1cnc2c(n[nH]c2n1)-c1ccc2nn(C)cc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615008
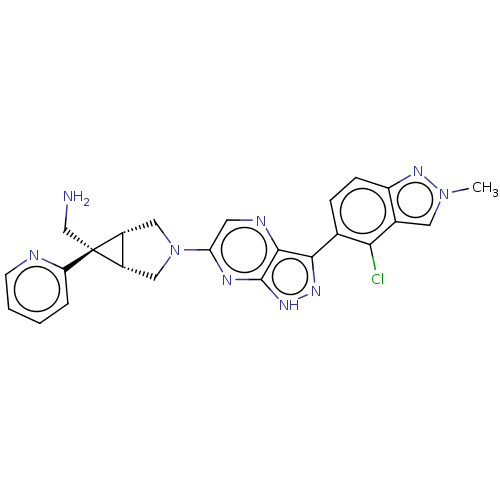
(CHEMBL5273026)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]2(CN)c1ccccn1)c1cnc2c(n[nH]c2n1)-c1ccc2nn(C)cc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615016
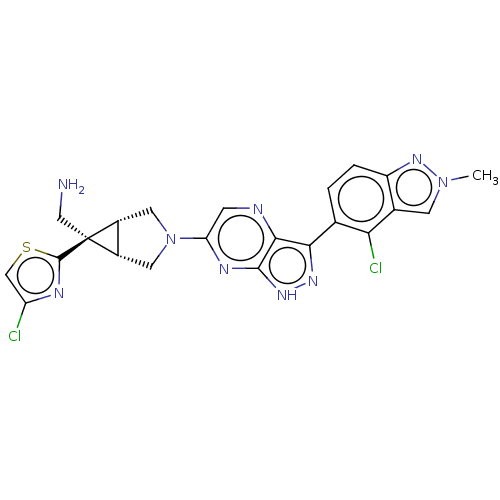
(CHEMBL5272403)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]2(CN)c1nc(Cl)cs1)c1cnc2c(n[nH]c2n1)-c1ccc2nn(C)cc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50592392
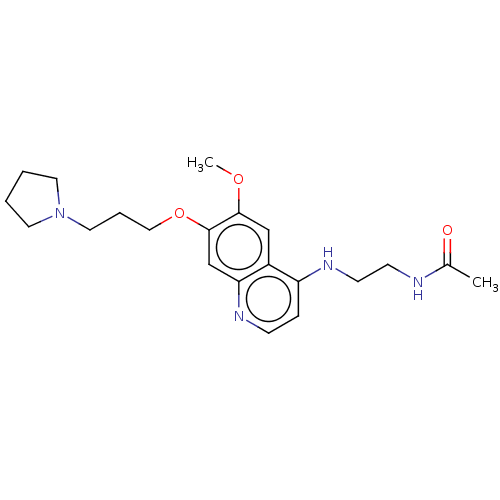
(CHEMBL5193402) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50592390

(CHEMBL5172085) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50446376

(CHEMBL3109630)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(N)C21CCC1 |t:19| Show InChI InChI=1S/C19H27N3O2/c1-23-16-12-14-15(21-18(20)19(14)6-4-7-19)13-17(16)24-11-5-10-22-8-2-3-9-22/h12-13H,2-11H2,1H3,(H2,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50614996
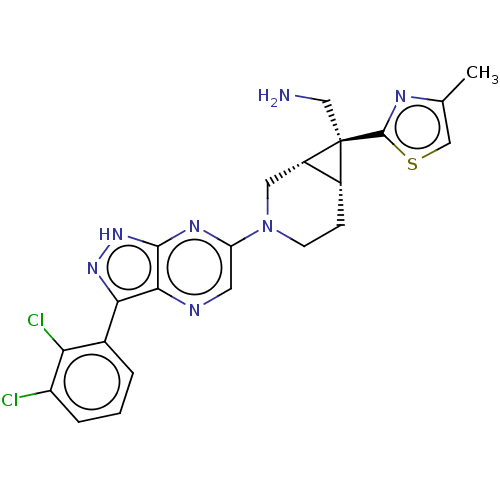
(CHEMBL5290513)Show SMILES [H][C@@]12CCN(C[C@]1([H])[C@@]2(CN)c1nc(C)cs1)c1cnc2c(n[nH]c2n1)-c1cccc(Cl)c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615003
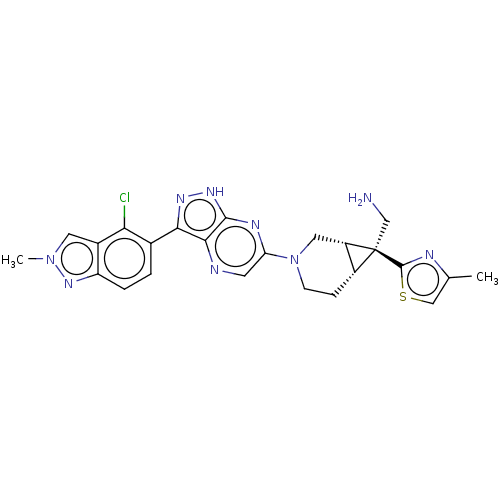
(CHEMBL5278658)Show SMILES [H][C@@]12CCN(C[C@]1([H])[C@@]2(CN)c1nc(C)cs1)c1cnc2c(n[nH]c2n1)-c1ccc2nn(C)cc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615014
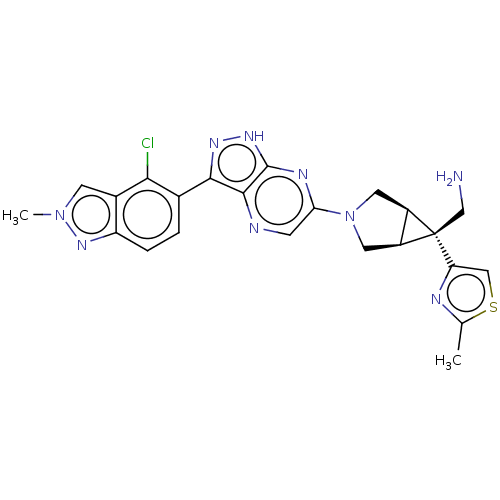
(CHEMBL5270851)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]2(CN)c1csc(C)n1)c1cnc2c(n[nH]c2n1)-c1ccc2nn(C)cc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171310
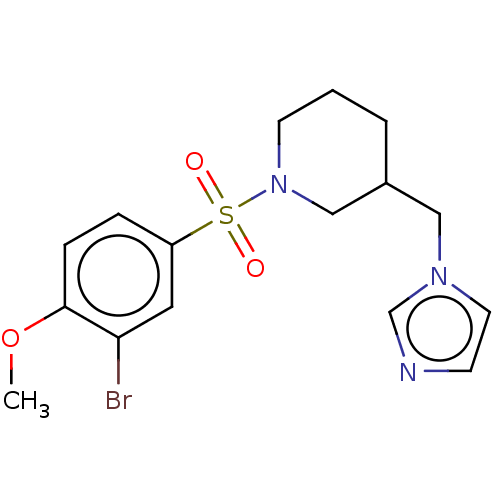
(CHEMBL3805814)Show InChI InChI=1S/C16H20BrN3O3S/c1-23-16-5-4-14(9-15(16)17)24(21,22)20-7-2-3-13(11-20)10-19-8-6-18-12-19/h4-6,8-9,12-13H,2-3,7,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615011
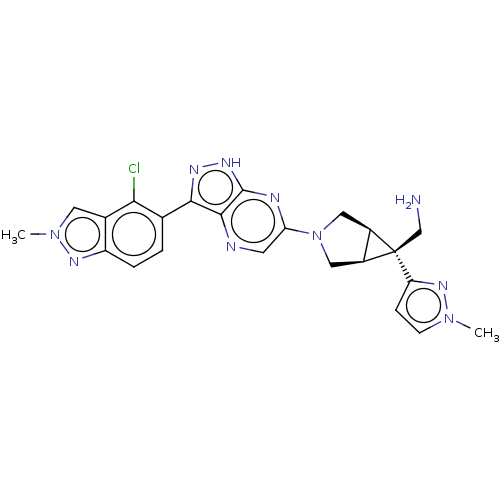
(CHEMBL5278669)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]2(CN)c1ccn(C)n1)c1cnc2c(n[nH]c2n1)-c1ccc2nn(C)cc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171231
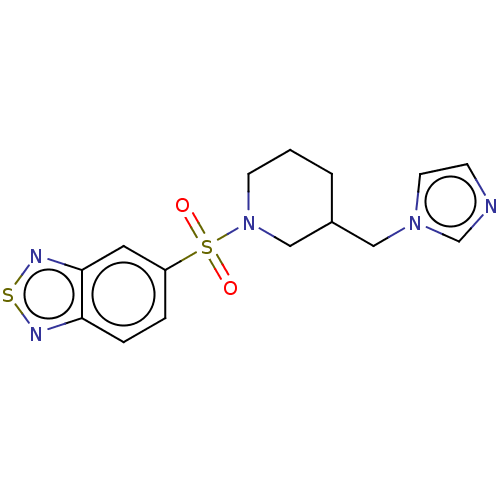
(CHEMBL3805851)Show InChI InChI=1S/C15H17N5O2S2/c21-24(22,13-3-4-14-15(8-13)18-23-17-14)20-6-1-2-12(10-20)9-19-7-5-16-11-19/h3-5,7-8,11-12H,1-2,6,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50614994
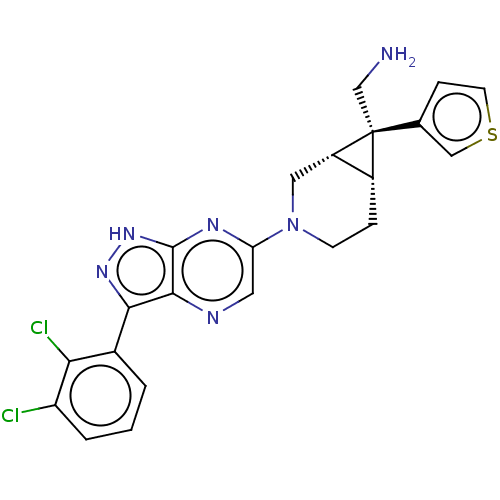
(CHEMBL5275479)Show SMILES [H][C@@]12CCN(C[C@]1([H])[C@@]2(CN)c1ccsc1)c1cnc2c(n[nH]c2n1)-c1cccc(Cl)c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50614988

(CHEMBL5269824)Show SMILES [H][C@@]12CCN(C[C@]1([H])[C@@]2(CN)c1nc(C)cs1)c1nc2nc(Sc3cccnc3C(F)(F)F)cnc2[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50614992
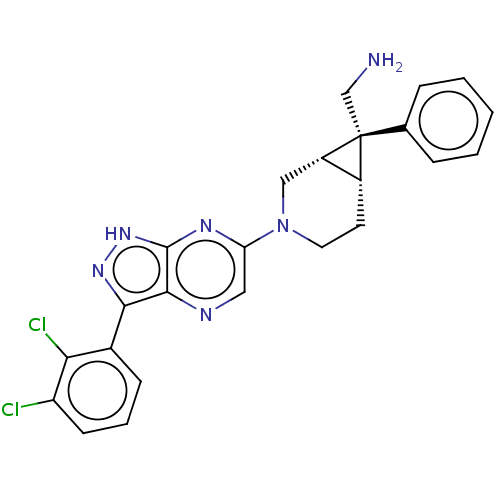
(CHEMBL5290180)Show SMILES [H][C@@]12CCN(C[C@]1([H])[C@@]2(CN)c1ccccc1)c1cnc2c(n[nH]c2n1)-c1cccc(Cl)c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615007
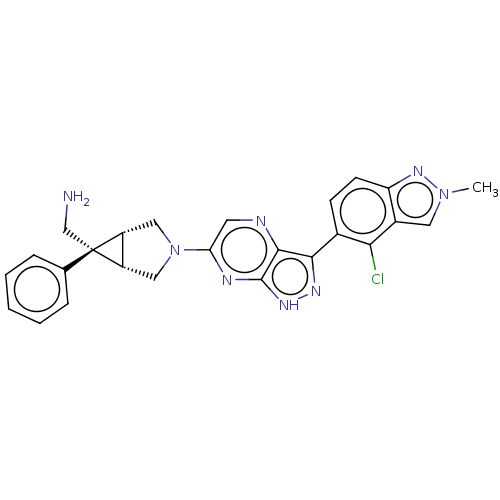
(CHEMBL5283063)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]2(CN)c1ccccc1)c1cnc2c(n[nH]c2n1)-c1ccc2nn(C)cc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171227

(CHEMBL3806301)Show SMILES [O-][N+](=O)c1cccc(Cl)c1Oc1cccc(c1)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C21H21ClN4O5S/c22-19-7-2-8-20(26(27)28)21(19)31-17-5-1-6-18(12-17)32(29,30)25-10-3-4-16(14-25)13-24-11-9-23-15-24/h1-2,5-9,11-12,15-16H,3-4,10,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50345657
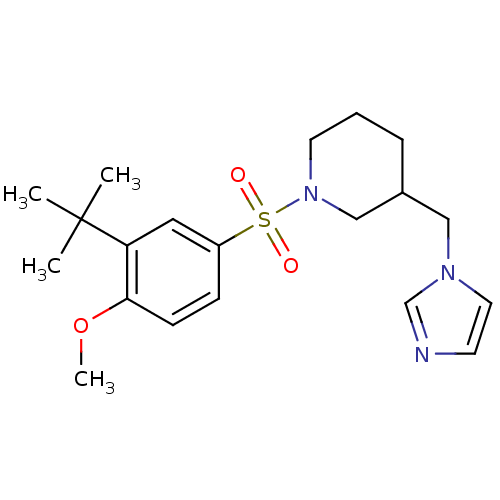
(CHEMBL1784801 | rac-3-((1H-imidazol-1-yl)methyl)-1...)Show SMILES COc1ccc(cc1C(C)(C)C)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C20H29N3O3S/c1-20(2,3)18-12-17(7-8-19(18)26-4)27(24,25)23-10-5-6-16(14-23)13-22-11-9-21-15-22/h7-9,11-12,15-16H,5-6,10,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615012
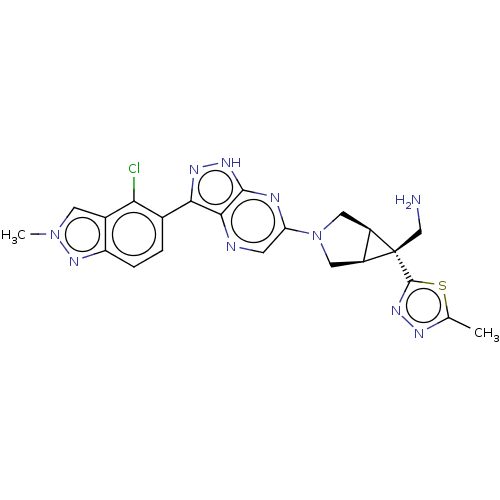
(CHEMBL5275866)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]2(CN)c1nnc(C)s1)c1cnc2c(n[nH]c2n1)-c1ccc2nn(C)cc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cyclooxygenase of human platelets was determined |
J Med Chem 30: 726-9 (1987)
BindingDB Entry DOI: 10.7270/Q2Q81G93 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50615017
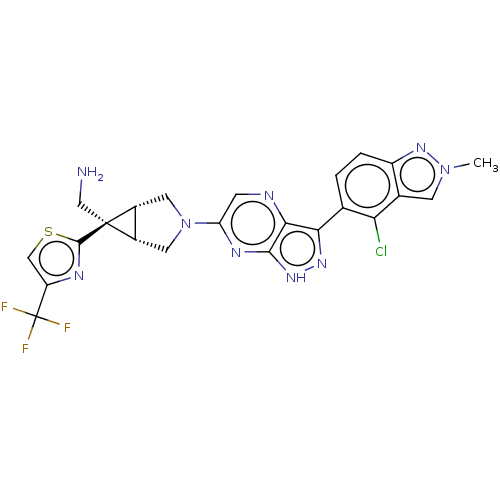
(CHEMBL5285837)Show SMILES [H][C@]12CN(C[C@@]1([H])[C@]2(CN)c1nc(cs1)C(F)(F)F)c1cnc2c(n[nH]c2n1)-c1ccc2nn(C)cc2c1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50615002

(CHEMBL5282278)Show SMILES [H][C@@]12CCN(C[C@]1([H])[C@@]2(CN)c1nc(C)cs1)c1cnc2c(n[nH]c2n1)-c1ccn2nccc2c1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171229
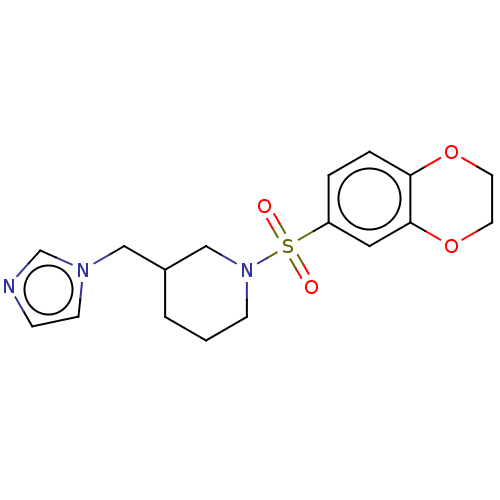
(CHEMBL3805211)Show InChI InChI=1S/C17H21N3O4S/c21-25(22,15-3-4-16-17(10-15)24-9-8-23-16)20-6-1-2-14(12-20)11-19-7-5-18-13-19/h3-5,7,10,13-14H,1-2,6,8-9,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171226
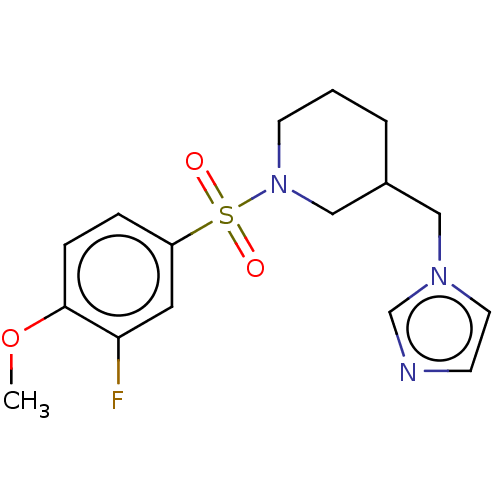
(CHEMBL3805173)Show InChI InChI=1S/C16H20FN3O3S/c1-23-16-5-4-14(9-15(16)17)24(21,22)20-7-2-3-13(11-20)10-19-8-6-18-12-19/h4-6,8-9,12-13H,2-3,7,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171228
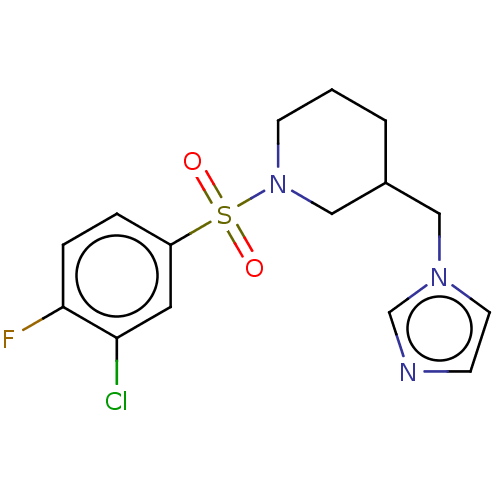
(CHEMBL3805481)Show InChI InChI=1S/C15H17ClFN3O2S/c16-14-8-13(3-4-15(14)17)23(21,22)20-6-1-2-12(10-20)9-19-7-5-18-11-19/h3-5,7-8,11-12H,1-2,6,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171390

(CHEMBL3805733)Show SMILES O=S(=O)(N1CCCC(Cn2ccnc2)C1)c1ccc(OC2CCCC2)cc1 Show InChI InChI=1S/C20H27N3O3S/c24-27(25,20-9-7-19(8-10-20)26-18-5-1-2-6-18)23-12-3-4-17(15-23)14-22-13-11-21-16-22/h7-11,13,16-18H,1-6,12,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171345
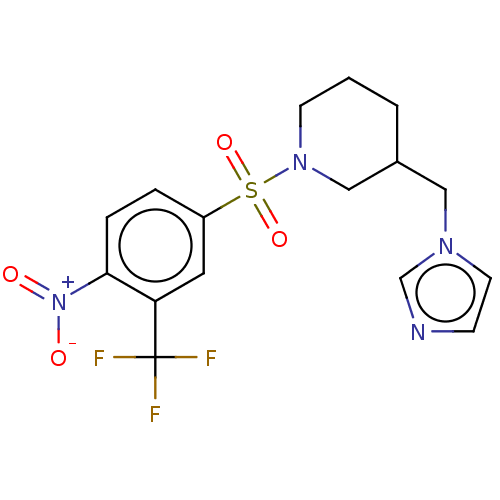
(CHEMBL3805008)Show SMILES [O-][N+](=O)c1ccc(cc1C(F)(F)F)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C16H17F3N4O4S/c17-16(18,19)14-8-13(3-4-15(14)23(24)25)28(26,27)22-6-1-2-12(10-22)9-21-7-5-20-11-21/h3-5,7-8,11-12H,1-2,6,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50592390

(CHEMBL5172085) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50614987

(CHEMBL5271480)Show SMILES [H][C@@]12CCN(C[C@]1([H])[C@@]2(CN)c1ccccn1)c1nc2nc(Sc3cccnc3C(F)(F)F)cnc2[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50592395
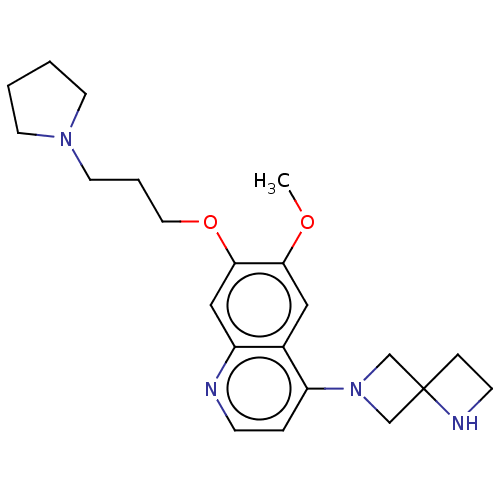
(CHEMBL5183771) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128858
BindingDB Entry DOI: 10.7270/Q2RX9H28 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171348

(CHEMBL3806075)Show SMILES COc1c(C)cc(cc1C)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C18H25N3O3S/c1-14-9-17(10-15(2)18(14)24-3)25(22,23)21-7-4-5-16(12-21)11-20-8-6-19-13-20/h6,8-10,13,16H,4-5,7,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171350

(CHEMBL3805539)Show SMILES Fc1cc(ccc1OC1CCCC1)S(=O)(=O)N1CCCC(Cn2ccnc2)C1 Show InChI InChI=1S/C20H26FN3O3S/c21-19-12-18(7-8-20(19)27-17-5-1-2-6-17)28(25,26)24-10-3-4-16(14-24)13-23-11-9-22-15-23/h7-9,11-12,15-17H,1-6,10,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50615001

(CHEMBL5267582)Show SMILES [H][C@@]12CCN(C[C@]1([H])[C@@]2(CN)c1nc(C)cs1)c1cnc2c(n[nH]c2n1)-c1ccc2nc(C)sc2c1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50171208
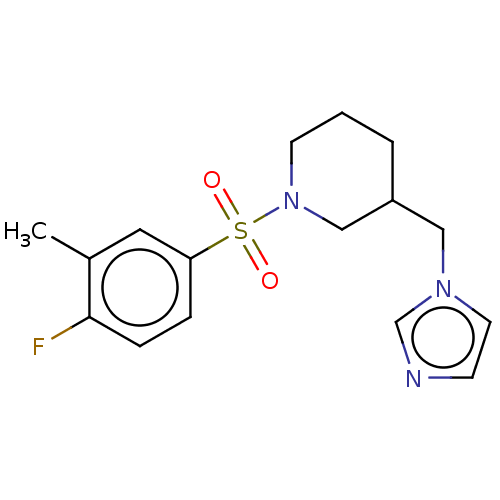
(CHEMBL3805310)Show InChI InChI=1S/C16H20FN3O2S/c1-13-9-15(4-5-16(13)17)23(21,22)20-7-2-3-14(11-20)10-19-8-6-18-12-19/h4-6,8-9,12,14H,2-3,7,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chieti 'G. d'Annunzio'
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human aromatase using 7-methoxy-4-trifluoromethyl coumarin as substrate measured at anoxic conditions by fluorescence analy... |
Bioorg Med Chem Lett 26: 3192-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.078
BindingDB Entry DOI: 10.7270/Q29C70CS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data