 Found 14 hits with Last Name = 'muzard' and Initial = 'm'
Found 14 hits with Last Name = 'muzard' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50370431
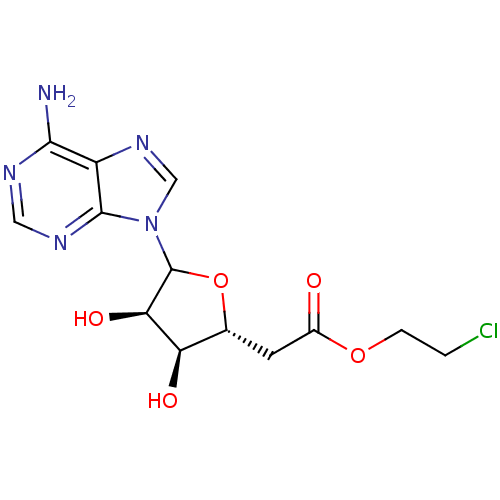
(CHEMBL610574)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](CC(=O)OCCCl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C13H16ClN5O5/c14-1-2-23-7(20)3-6-9(21)10(22)13(24-6)19-5-18-8-11(15)16-4-17-12(8)19/h4-6,9-10,13,21-22H,1-3H2,(H2,15,16,17)/t6-,9-,10-,13?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 6519
Curated by ChEMBL
| Assay Description
Inhibition of human placental AdoHcy hydrolase |
Bioorg Med Chem Lett 14: 5799-802 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.050
BindingDB Entry DOI: 10.7270/Q2RN38NZ |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM84862
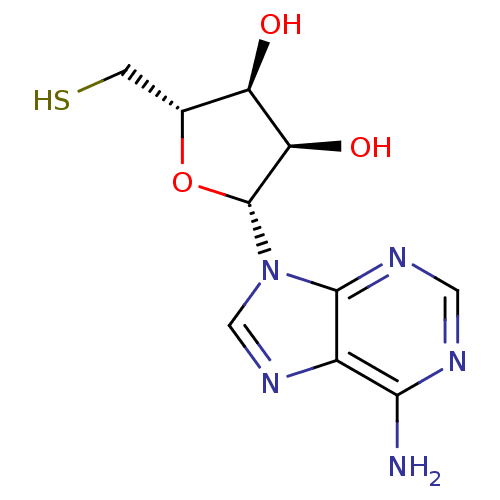
(5'-Deoxy-5'-thiodenosine scaffold, 6)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CS)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H13N5O3S/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(1-19)18-10/h2-4,6-7,10,16-17,19H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 6519
Curated by ChEMBL
| Assay Description
Binding affinity for human placental S-adenosyl-homocysteine hydrolase |
Bioorg Med Chem Lett 14: 5803-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.051
BindingDB Entry DOI: 10.7270/Q2MW2HX7 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50370432
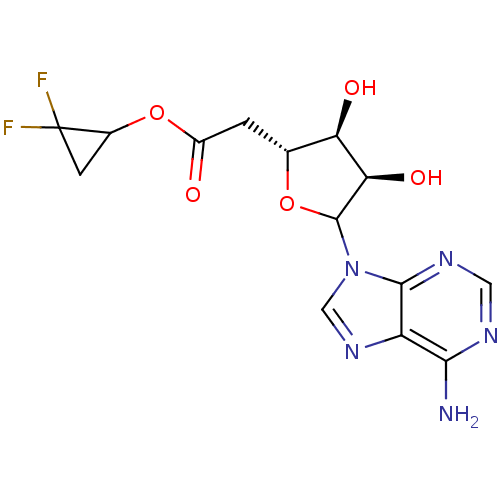
(CHEMBL611108)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](CC(=O)OC2CC2(F)F)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H15F2N5O5/c15-14(16)2-6(14)26-7(22)1-5-9(23)10(24)13(25-5)21-4-20-8-11(17)18-3-19-12(8)21/h3-6,9-10,13,23-24H,1-2H2,(H2,17,18,19)/t5-,6?,9-,10-,13?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 6519
Curated by ChEMBL
| Assay Description
Inhibition of human placental AdoHcy hydrolase |
Bioorg Med Chem Lett 14: 5799-802 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.050
BindingDB Entry DOI: 10.7270/Q2RN38NZ |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50182453
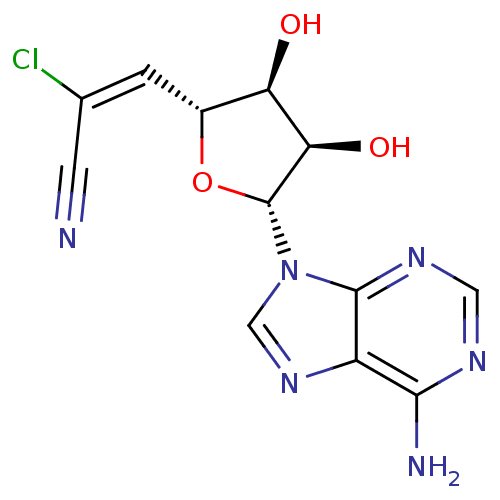
((E)-6'-chloro-6'-cyano-5',6'-didehydro-6'-deoxyhom...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](\C=C(\Cl)C#N)[C@@H](O)[C@H]1O Show InChI InChI=1S/C12H11ClN6O3/c13-5(2-14)1-6-8(20)9(21)12(22-6)19-4-18-7-10(15)16-3-17-11(7)19/h1,3-4,6,8-9,12,20-21H,(H2,15,16,17)/b5-1+/t6-,8-,9-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against AdoHcy Hydrolase |
J Med Chem 49: 1223-6 (2006)
Article DOI: 10.1021/jm051023x
BindingDB Entry DOI: 10.7270/Q2RN37FV |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50370433
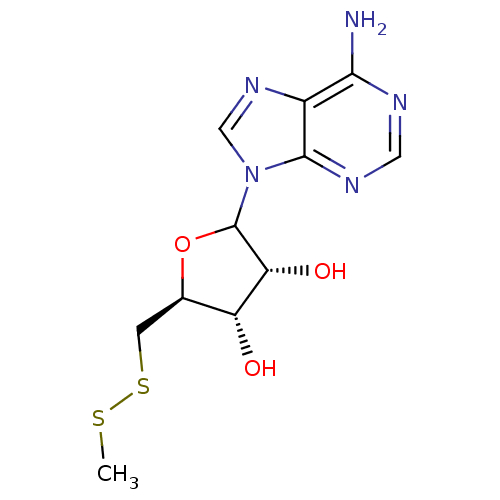
(CHEMBL610838)Show SMILES CSSC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C11H15N5O3S2/c1-20-21-2-5-7(17)8(18)11(19-5)16-4-15-6-9(12)13-3-14-10(6)16/h3-5,7-8,11,17-18H,2H2,1H3,(H2,12,13,14)/t5-,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 6519
Curated by ChEMBL
| Assay Description
Binding affinity for human placental S-adenosyl-homocysteine hydrolase |
Bioorg Med Chem Lett 14: 5803-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.051
BindingDB Entry DOI: 10.7270/Q2MW2HX7 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50370435
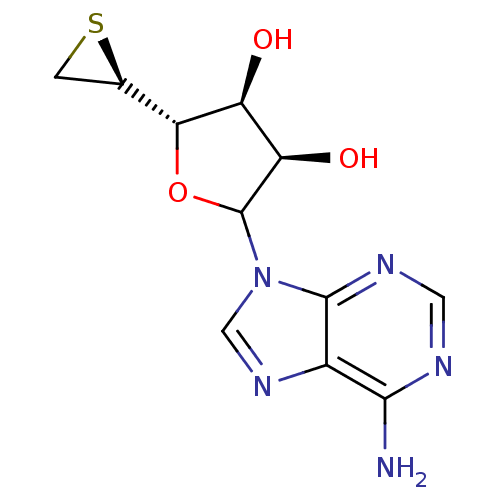
(CHEMBL610303)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H]([C@H]2CS2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13N5O3S/c12-9-5-10(14-2-13-9)16(3-15-5)11-7(18)6(17)8(19-11)4-1-20-4/h2-4,6-8,11,17-18H,1H2,(H2,12,13,14)/t4-,6+,7-,8-,11?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 6519
Curated by ChEMBL
| Assay Description
Binding affinity for human placental S-adenosyl-homocysteine hydrolase |
Bioorg Med Chem Lett 14: 5803-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.051
BindingDB Entry DOI: 10.7270/Q2MW2HX7 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50485112
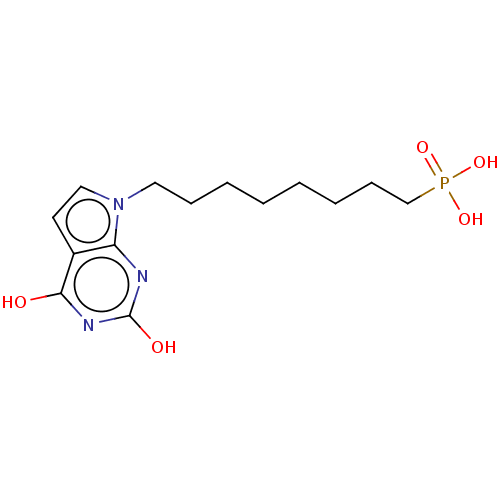
(CHEMBL2030733)Show InChI InChI=1S/C14H22N3O5P/c18-13-11-7-9-17(12(11)15-14(19)16-13)8-5-3-1-2-4-6-10-23(20,21)22/h7,9H,1-6,8,10H2,(H2,20,21,22)(H2,15,16,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Competitive inhibition of Escherichia coli thymidine phosphorylase incubated for 20 mins at room temperature followed by 5 mins incubation at 90 degC... |
J Med Chem 55: 2758-68 (2012)
Article DOI: 10.1021/jm201694y
BindingDB Entry DOI: 10.7270/Q2GT5R1P |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50485113

(CHEMBL2030728)Show SMILES Cc1cn(Cc2cn(CCCCC(F)(F)P(O)(O)=O)nn2)c(=O)[nH]c1=O Show InChI InChI=1S/C13H18F2N5O5P/c1-9-6-19(12(22)16-11(9)21)7-10-8-20(18-17-10)5-3-2-4-13(14,15)26(23,24)25/h6,8H,2-5,7H2,1H3,(H,16,21,22)(H2,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Competitive inhibition of Escherichia coli thymidine phosphorylase incubated for 20 mins at room temperature followed by 5 mins incubation at 90 degC... |
J Med Chem 55: 2758-68 (2012)
Article DOI: 10.1021/jm201694y
BindingDB Entry DOI: 10.7270/Q2GT5R1P |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50370430
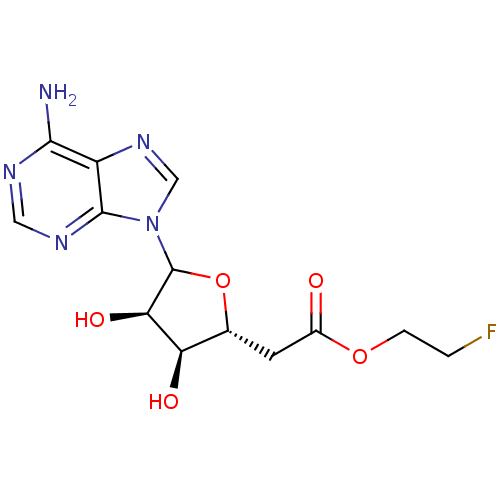
(CHEMBL610300)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](CC(=O)OCCF)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C13H16FN5O5/c14-1-2-23-7(20)3-6-9(21)10(22)13(24-6)19-5-18-8-11(15)16-4-17-12(8)19/h4-6,9-10,13,21-22H,1-3H2,(H2,15,16,17)/t6-,9-,10-,13?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 6519
Curated by ChEMBL
| Assay Description
Inhibition of human placental AdoHcy hydrolase |
Bioorg Med Chem Lett 14: 5799-802 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.050
BindingDB Entry DOI: 10.7270/Q2RN38NZ |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50370436
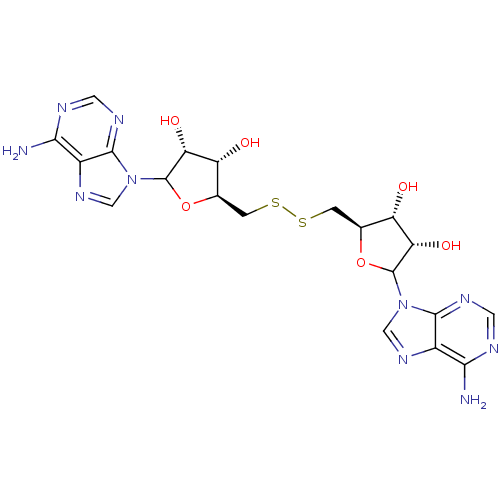
(CHEMBL611107)Show SMILES Nc1ncnc2n(cnc12)C1O[C@@H](CSSC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C20H24N10O6S2/c21-15-9-17(25-3-23-15)29(5-27-9)19-13(33)11(31)7(35-19)1-37-38-2-8-12(32)14(34)20(36-8)30-6-28-10-16(22)24-4-26-18(10)30/h3-8,11-14,19-20,31-34H,1-2H2,(H2,21,23,25)(H2,22,24,26)/t7-,8+,11-,12+,13-,14+,19?,20? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 6519
Curated by ChEMBL
| Assay Description
Binding affinity for human placental S-adenosyl-homocysteine hydrolase |
Bioorg Med Chem Lett 14: 5803-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.051
BindingDB Entry DOI: 10.7270/Q2MW2HX7 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50370434
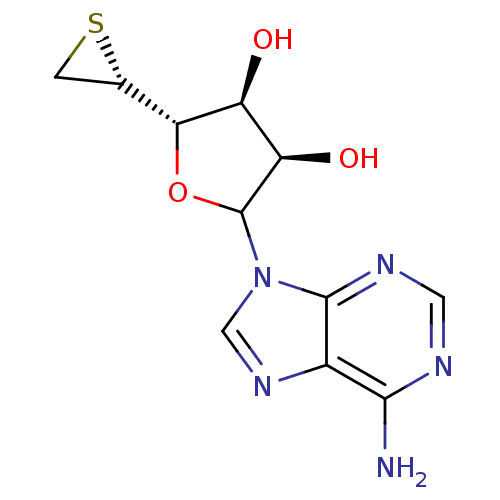
(CHEMBL610588)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H]([C@@H]2CS2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H13N5O3S/c12-9-5-10(14-2-13-9)16(3-15-5)11-7(18)6(17)8(19-11)4-1-20-4/h2-4,6-8,11,17-18H,1H2,(H2,12,13,14)/t4-,6-,7+,8+,11?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 6519
Curated by ChEMBL
| Assay Description
Binding affinity for human placental S-adenosyl-homocysteine hydrolase |
Bioorg Med Chem Lett 14: 5803-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.051
BindingDB Entry DOI: 10.7270/Q2MW2HX7 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50485111
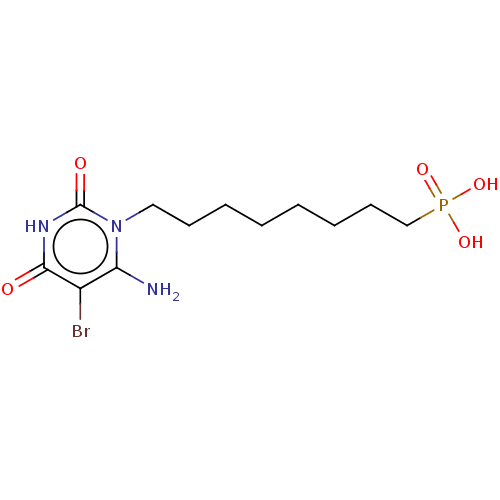
(CHEMBL368694)Show InChI InChI=1S/C12H21BrN3O5P/c13-9-10(14)16(12(18)15-11(9)17)7-5-3-1-2-4-6-8-22(19,20)21/h1-8,14H2,(H,15,17,18)(H2,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie
Curated by ChEMBL
| Assay Description
Competitive inhibition of Escherichia coli thymidine phosphorylase incubated for 20 mins at room temperature followed by 5 mins incubation at 90 degC... |
J Med Chem 55: 2758-68 (2012)
Article DOI: 10.1021/jm201694y
BindingDB Entry DOI: 10.7270/Q2GT5R1P |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Rattus norvegicus) | BDBM50366378

(CHEMBL604650)Show SMILES Nc1ncnc2n(cnc12)C1O[C@](F)(CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H12FN5O4/c11-10(1-17)6(19)5(18)9(20-10)16-3-15-4-7(12)13-2-14-8(4)16/h2-3,5-6,9,17-19H,1H2,(H2,12,13,14)/t5-,6+,9?,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant against rat liver S-adenosyl-L-homocysteine hydrolase |
Bioorg Med Chem Lett 5: 1455-1460 (1995)
Article DOI: 10.1016/0960-894X(95)00256-S
BindingDB Entry DOI: 10.7270/Q2C53MBT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50370429
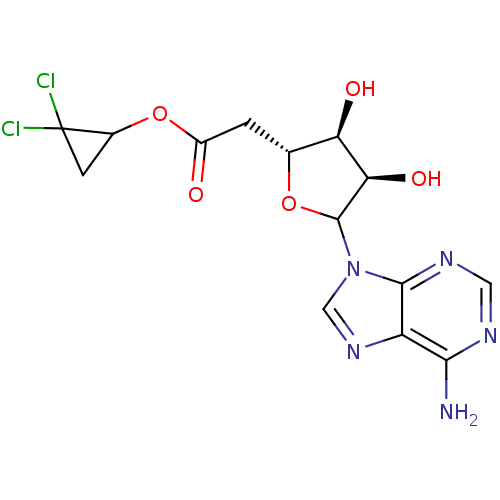
(CHEMBL610573)Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](CC(=O)OC2CC2(Cl)Cl)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H15Cl2N5O5/c15-14(16)2-6(14)26-7(22)1-5-9(23)10(24)13(25-5)21-4-20-8-11(17)18-3-19-12(8)21/h3-6,9-10,13,23-24H,1-2H2,(H2,17,18,19)/t5-,6?,9-,10-,13?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 6519
Curated by ChEMBL
| Assay Description
Inhibition of human placental AdoHcy hydrolase |
Bioorg Med Chem Lett 14: 5799-802 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.050
BindingDB Entry DOI: 10.7270/Q2RN38NZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data