 Found 127 hits with Last Name = 'neuenschwander' and Initial = 'm'
Found 127 hits with Last Name = 'neuenschwander' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357735
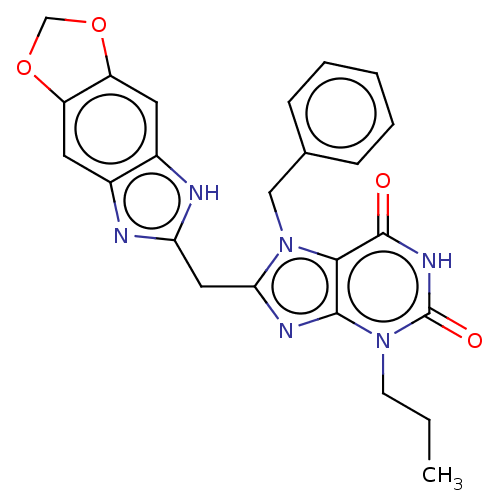
(KM-05-193/(I-209) | US10214530, Example 27)Show SMILES CCCn1c2nc(Cc3nc4cc5OCOc5cc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O Show InChI InChI=1S/C24H22N6O4/c1-2-8-29-22-21(23(31)28-24(29)32)30(12-14-6-4-3-5-7-14)20(27-22)11-19-25-15-9-17-18(34-13-33-17)10-16(15)26-19/h3-7,9-10H,2,8,11-13H2,1H3,(H,25,26)(H,28,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357737
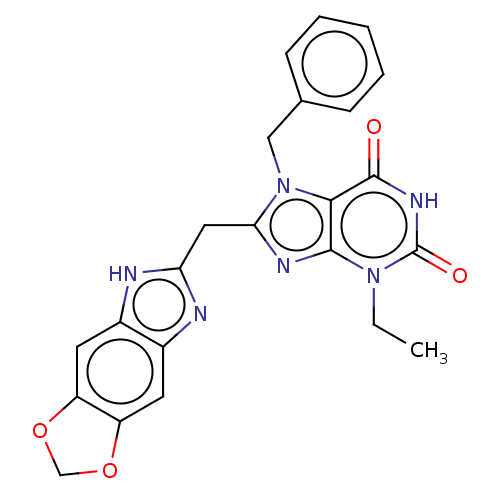
(KM-05-166/(I-206) | US10214530, Example 24)Show SMILES CCn1c2nc(Cc3nc4cc5OCOc5cc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O Show InChI InChI=1S/C23H20N6O4/c1-2-28-21-20(22(30)27-23(28)31)29(11-13-6-4-3-5-7-13)19(26-21)10-18-24-14-8-16-17(33-12-32-16)9-15(14)25-18/h3-9H,2,10-12H2,1H3,(H,24,25)(H,27,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357739

(AG-01-128/(I-202) | US10214530, Example 18)Show SMILES CCn1c2nc(Cc3nc4ccccc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O Show InChI InChI=1S/C22H20N6O2/c1-2-27-20-19(21(29)26-22(27)30)28(13-14-8-4-3-5-9-14)18(25-20)12-17-23-15-10-6-7-11-16(15)24-17/h3-11H,2,12-13H2,1H3,(H,23,24)(H,26,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM50598895

(CHEMBL5172829)Show SMILES CCOc1ccc2[nH]c(Cc3nc4n(CC)c(=O)[nH]c(=O)c4n3Cc3ccccc3)nc2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM50598894

(CHEMBL5197055)Show SMILES CCCn1c2nc(Cc3nc4cc(O)ccc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357736

(MW-01-157/(I-207) | US10214530, Example 25)Show SMILES CCCn1c2nc(Cc3nc4ccccc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O Show InChI InChI=1S/C23H22N6O2/c1-2-12-28-21-20(22(30)27-23(28)31)29(14-15-8-4-3-5-9-15)19(26-21)13-18-24-16-10-6-7-11-17(16)25-18/h3-11H,2,12-14H2,1H3,(H,24,25)(H,27,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50556732
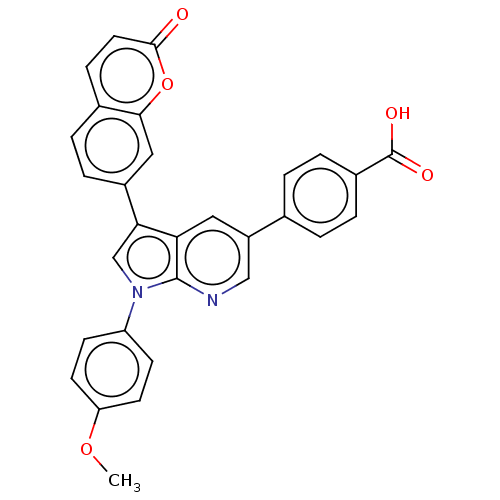
(CHEMBL4750435)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc3ccc(=O)oc3c2)c2cc(cnc12)-c1ccc(cc1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357741
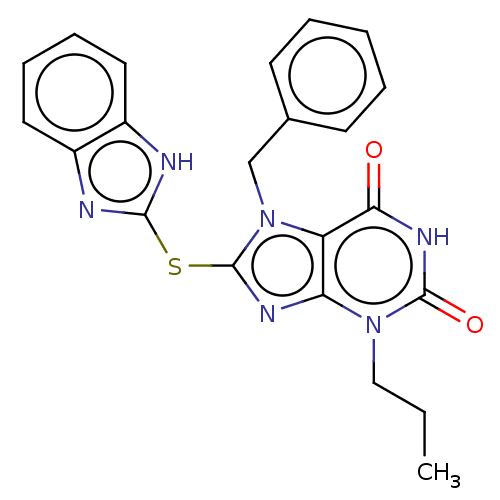
(MW-01-153/(I-47) | US10214530, Example 53)Show SMILES CCCn1c2nc(Sc3nc4ccccc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O Show InChI InChI=1S/C22H20N6O2S/c1-2-12-27-18-17(19(29)26-21(27)30)28(13-14-8-4-3-5-9-14)22(25-18)31-20-23-15-10-6-7-11-16(15)24-20/h3-11H,2,12-13H2,1H3,(H,23,24)(H,26,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357743

(MW-01-139/(I-46) | US10214530, Example 52)Show SMILES CCn1c2nc(Sc3nc4ccccc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O Show InChI InChI=1S/C21H18N6O2S/c1-2-26-17-16(18(28)25-20(26)29)27(12-13-8-4-3-5-9-13)21(24-17)30-19-22-14-10-6-7-11-15(14)23-19/h3-11H,2,12H2,1H3,(H,22,23)(H,25,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM50598896
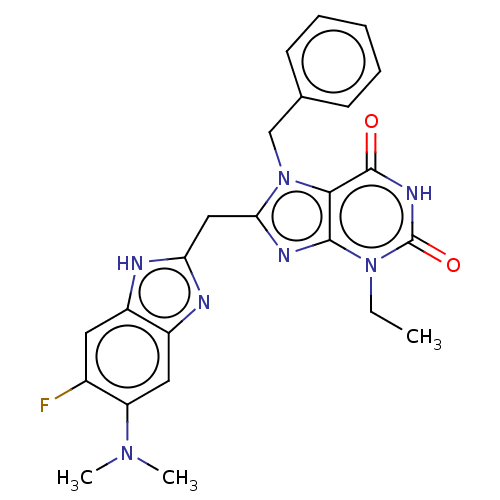
(CHEMBL5176333)Show SMILES CCn1c2nc(Cc3nc4cc(N(C)C)c(F)cc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357740

(KM-05-139/(I-23) | US10214530, Example 23)Show SMILES O=c1[nH]c(=O)c2n(Cc3ccccc3)c(Cc3nc4cc5OCOc5cc4[nH]3)nc2n1C1CC1 Show InChI InChI=1S/C24H20N6O4/c31-23-21-22(30(14-6-7-14)24(32)28-23)27-20(29(21)11-13-4-2-1-3-5-13)10-19-25-15-8-17-18(34-12-33-17)9-16(15)26-19/h1-5,8-9,14H,6-7,10-12H2,(H,25,26)(H,28,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357738

(KM-05-130/(I-19) | US10214530, Example 19)Show SMILES Oc1ccc2nc(Cc3nc4n(C5CC5)c(=O)[nH]c(=O)c4n3Cc3ccccc3)[nH]c2c1 Show InChI InChI=1S/C23H20N6O3/c30-15-8-9-16-17(10-15)25-18(24-16)11-19-26-21-20(28(19)12-13-4-2-1-3-5-13)22(31)27-23(32)29(21)14-6-7-14/h1-5,8-10,14,30H,6-7,11-12H2,(H,24,25)(H,27,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50342004

(4-(2-(3-(4-nitrophenyl)-5-oxo-1-phenyl-1H-pyrazol-...)Show SMILES OS(=O)(=O)c1ccc(cc1)N=Nc1c([nH]n(-c2ccccc2)c1=O)-c1ccc(cc1)[N+]([O-])=O |w:10.10| Show InChI InChI=1S/C21H15N5O6S/c27-21-20(23-22-15-8-12-18(13-9-15)33(30,31)32)19(14-6-10-17(11-7-14)26(28)29)24-25(21)16-4-2-1-3-5-16/h1-13,24H,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM50598897
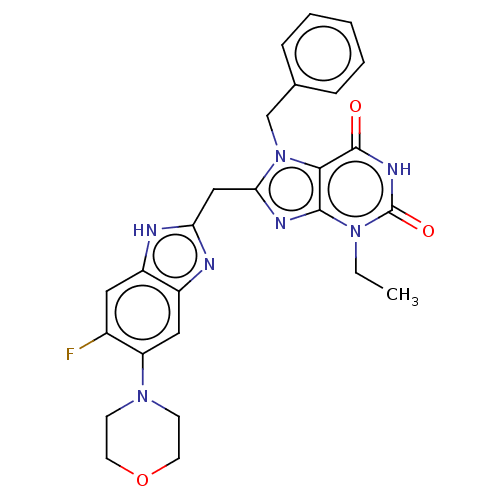
(CHEMBL5174736)Show SMILES CCn1c2nc(Cc3nc4cc(N5CCOCC5)c(F)cc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50556755
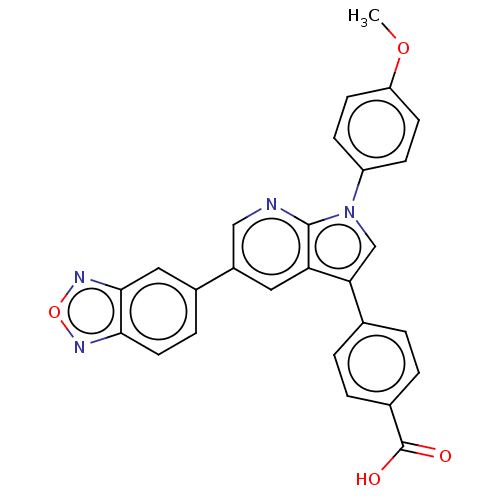
(CHEMBL4763014)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc(cc2)C(O)=O)c2cc(cnc12)-c1ccc2nonc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357744

(KM-05-80/(I-1) | US10214530, Example 1)Show SMILES O=c1[nH]c(=O)c2n(Cc3ccccc3)c(Cc3nc4ccccc4[nH]3)nc2n1C1CC1 Show InChI InChI=1S/C23H20N6O2/c30-22-20-21(29(15-10-11-15)23(31)27-22)26-19(28(20)13-14-6-2-1-3-7-14)12-18-24-16-8-4-5-9-17(16)25-18/h1-9,15H,10-13H2,(H,24,25)(H,27,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357745
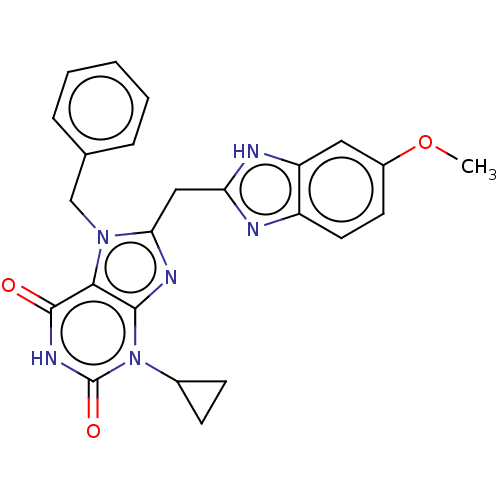
(KM-05-125/(I-13) | US10214530, Example 13)Show SMILES COc1ccc2nc(Cc3nc4n(C5CC5)c(=O)[nH]c(=O)c4n3Cc3ccccc3)[nH]c2c1 Show InChI InChI=1S/C24H22N6O3/c1-33-16-9-10-17-18(11-16)26-19(25-17)12-20-27-22-21(29(20)13-14-5-3-2-4-6-14)23(31)28-24(32)30(22)15-7-8-15/h2-6,9-11,15H,7-8,12-13H2,1H3,(H,25,26)(H,28,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50556754
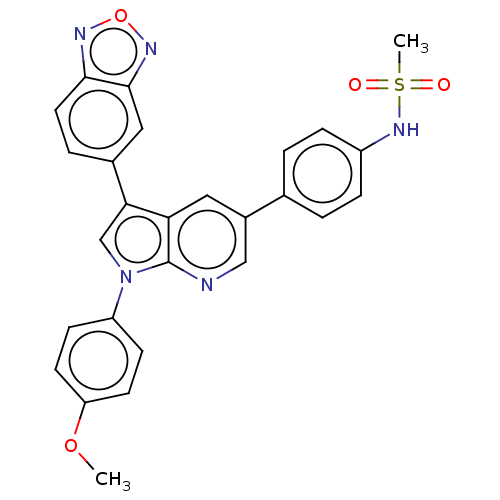
(CHEMBL4781443)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc3nonc3c2)c2cc(cnc12)-c1ccc(NS(C)(=O)=O)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357748

(KM-05-89/(I-2) | US10214530, Example 2)Show SMILES O=c1[nH]c(=O)c2n(Cc3ccccc3)c(Cc3nc4cc(ccc4[nH]3)N3CCOCC3)nc2n1C1CC1 Show InChI InChI=1S/C27H27N7O3/c35-26-24-25(34(18-6-7-18)27(36)31-26)30-23(33(24)16-17-4-2-1-3-5-17)15-22-28-20-9-8-19(14-21(20)29-22)32-10-12-37-13-11-32/h1-5,8-9,14,18H,6-7,10-13,15-16H2,(H,28,29)(H,31,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50556737

(CHEMBL4742016)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc(cc2)C(F)(F)F)c2cc(cnc12)-c1ccc(cc1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 12
(Homo sapiens (Human)) | BDBM50556732

(CHEMBL4750435)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc3ccc(=O)oc3c2)c2cc(cnc12)-c1ccc(cc1)C(O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN12 (unknown origin) pre-incubated for 20 mins followed by fluorescence substrate addition and measured after 120 mins by DiFMUP ass... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50556732

(CHEMBL4750435)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc3ccc(=O)oc3c2)c2cc(cnc12)-c1ccc(cc1)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN6 (unknown origin) pre-incubated for 20 mins followed by fluorescence substrate addition and measured after 120 mins by DiFMUP assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50556733
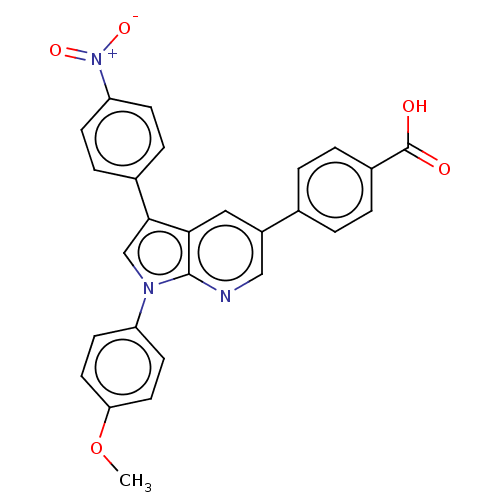
(CHEMBL4755222)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc(cc2)[N+]([O-])=O)c2cc(cnc12)-c1ccc(cc1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 7
(Homo sapiens (Human)) | BDBM50556732

(CHEMBL4750435)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc3ccc(=O)oc3c2)c2cc(cnc12)-c1ccc(cc1)C(O)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN7 (unknown origin) pre-incubated for 20 mins followed by fluorescence substrate addition and measured after 120 mins by DiFMUP assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357750

(KM-05-135/(I-15) | US10214530, Example 15)Show SMILES OCCOc1ccc2nc(Cc3nc4n(C5CC5)c(=O)[nH]c(=O)c4n3Cc3ccccc3)[nH]c2c1 Show InChI InChI=1S/C25H24N6O4/c32-10-11-35-17-8-9-18-19(12-17)27-20(26-18)13-21-28-23-22(30(21)14-15-4-2-1-3-5-15)24(33)29-25(34)31(23)16-6-7-16/h1-5,8-9,12,16,32H,6-7,10-11,13-14H2,(H,26,27)(H,29,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50556748
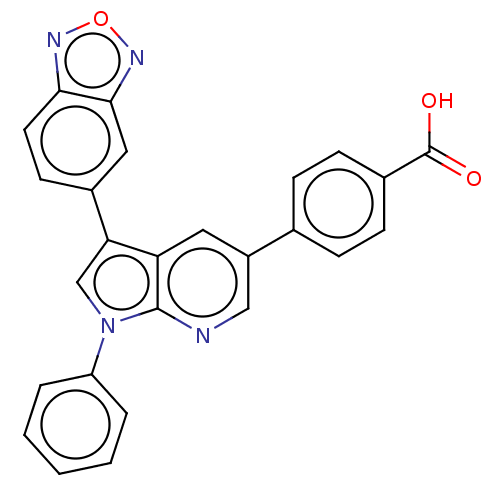
(CHEMBL4741433)Show SMILES OC(=O)c1ccc(cc1)-c1cnc2n(cc(-c3ccc4nonc4c3)c2c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357751

(KM-05-128/(I-17) | US10214530, Example 17)Show SMILES COC(=O)c1ccc2nc(Cc3nc4n(C5CC5)c(=O)[nH]c(=O)c4n3Cc3ccccc3)[nH]c2c1 Show InChI InChI=1S/C25H22N6O4/c1-35-24(33)15-7-10-17-18(11-15)27-19(26-17)12-20-28-22-21(30(20)13-14-5-3-2-4-6-14)23(32)29-25(34)31(22)16-8-9-16/h2-7,10-11,16H,8-9,12-13H2,1H3,(H,26,27)(H,29,32,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357754

(KM-05-60/(I-10) | US10214530, Example 10)Show SMILES Cn1c2nc(Cc3nc4cc(NC(=O)CC[C@H](N)C(O)=O)ccc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357760
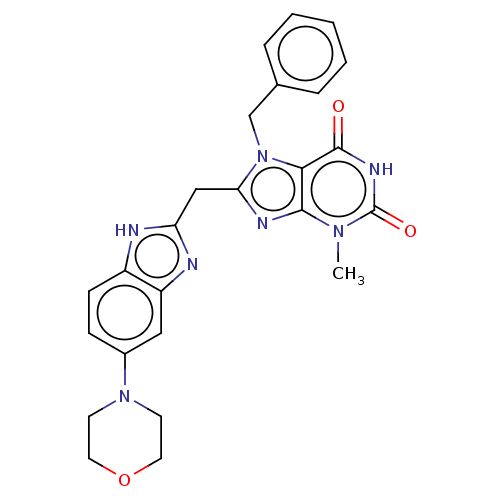
(KM-05-50/(I-4) | US10214530, Example 4)Show SMILES Cn1c2nc(Cc3nc4cc(ccc4[nH]3)N3CCOCC3)n(Cc3ccccc3)c2c(=O)[nH]c1=O Show InChI InChI=1S/C25H25N7O3/c1-30-23-22(24(33)29-25(30)34)32(15-16-5-3-2-4-6-16)21(28-23)14-20-26-18-8-7-17(13-19(18)27-20)31-9-11-35-12-10-31/h2-8,13H,9-12,14-15H2,1H3,(H,26,27)(H,29,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50556732

(CHEMBL4750435)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc3ccc(=O)oc3c2)c2cc(cnc12)-c1ccc(cc1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PP2A alpha (unknown origin) pre-incubated for 20 mins followed by fluorescence substrate addition and measured after 120 mins by DiFMUP... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357756

(KM480/(I-41) | US10214530, Example 47)Show SMILES Cn1c2nc(Sc3nc4cc(NC(=O)CC[C@H](N)C(O)=O)ccc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357752

(KM-05-126/(I-12) | US10214530, Example 12)Show SMILES Fc1ccc2nc(Cc3nc4n(C5CC5)c(=O)[nH]c(=O)c4n3Cc3ccccc3)[nH]c2c1 Show InChI InChI=1S/C23H19FN6O2/c24-14-6-9-16-17(10-14)26-18(25-16)11-19-27-21-20(29(19)12-13-4-2-1-3-5-13)22(31)28-23(32)30(21)15-7-8-15/h1-6,9-10,15H,7-8,11-12H2,(H,25,26)(H,28,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50556735
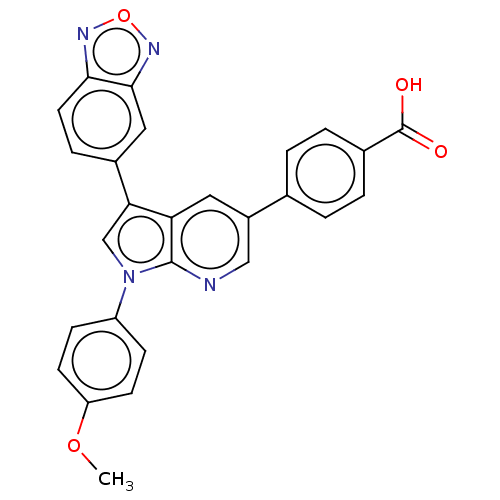
(CHEMBL4747136)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc3nonc3c2)c2cc(cnc12)-c1ccc(cc1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 1
(Homo sapiens (Human)) | BDBM50598894

(CHEMBL5197055)Show SMILES CCCn1c2nc(Cc3nc4cc(O)ccc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 1
(Homo sapiens (Human)) | BDBM357735

(KM-05-193/(I-209) | US10214530, Example 27)Show SMILES CCCn1c2nc(Cc3nc4cc5OCOc5cc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O Show InChI InChI=1S/C24H22N6O4/c1-2-8-29-22-21(23(31)28-24(29)32)30(12-14-6-4-3-5-7-14)20(27-22)11-19-25-15-9-17-18(34-13-33-17)10-16(15)26-19/h3-7,9-10H,2,8,11-13H2,1H3,(H,25,26)(H,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptophan 5-hydroxylase 1
(Homo sapiens (Human)) | BDBM357736

(MW-01-157/(I-207) | US10214530, Example 25)Show SMILES CCCn1c2nc(Cc3nc4ccccc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O Show InChI InChI=1S/C23H22N6O2/c1-2-12-28-21-20(22(30)27-23(28)31)29(14-15-8-4-3-5-9-15)19(26-21)13-18-24-16-10-6-7-11-17(16)25-18/h3-11H,2,12-14H2,1H3,(H,24,25)(H,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 1
(Homo sapiens (Human)) | BDBM357737

(KM-05-166/(I-206) | US10214530, Example 24)Show SMILES CCn1c2nc(Cc3nc4cc5OCOc5cc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O Show InChI InChI=1S/C23H20N6O4/c1-2-28-21-20(22(30)27-23(28)31)29(11-13-6-4-3-5-7-13)19(26-21)10-18-24-14-8-16-17(33-12-32-16)9-15(14)25-18/h3-9H,2,10-12H2,1H3,(H,24,25)(H,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50556743
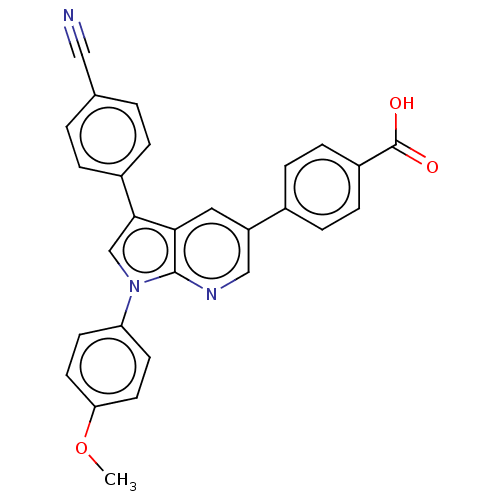
(CHEMBL4783825)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc(cc2)C#N)c2cc(cnc12)-c1ccc(cc1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50342003
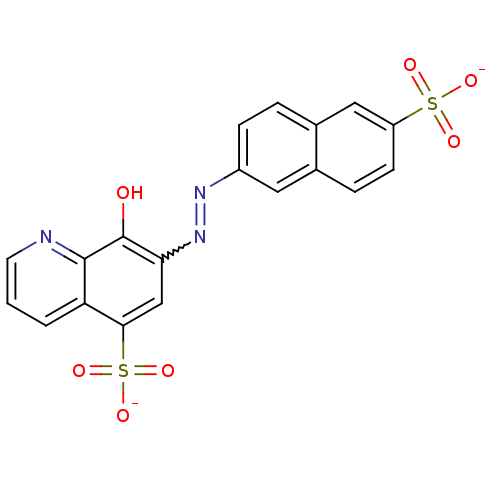
(CHEMBL501993 | NSC-87877 | disodium 8-hydroxy-7-[(...)Show SMILES Oc1c(cc(c2cccnc12)S([O-])(=O)=O)N=Nc1ccc2cc(ccc2c1)S([O-])(=O)=O |w:15.16| Show InChI InChI=1S/C19H13N3O7S2/c23-19-16(10-17(31(27,28)29)15-2-1-7-20-18(15)19)22-21-13-5-3-12-9-14(30(24,25)26)6-4-11(12)8-13/h1-10,23H,(H,24,25,26)(H,27,28,29)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50556736
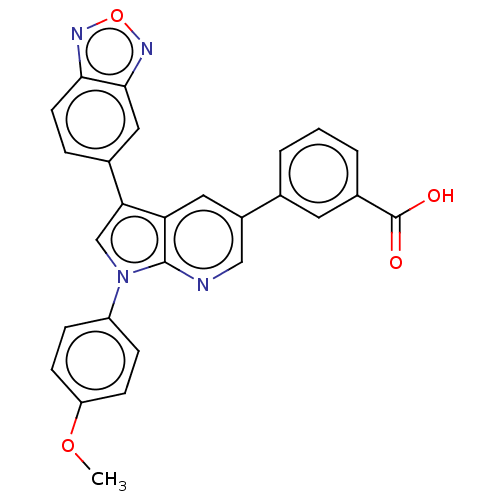
(CHEMBL4749307)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc3nonc3c2)c2cc(cnc12)-c1cccc(c1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 1
(Homo sapiens (Human)) | BDBM357738

(KM-05-130/(I-19) | US10214530, Example 19)Show SMILES Oc1ccc2nc(Cc3nc4n(C5CC5)c(=O)[nH]c(=O)c4n3Cc3ccccc3)[nH]c2c1 Show InChI InChI=1S/C23H20N6O3/c30-15-8-9-16-17(10-15)25-18(24-16)11-19-26-21-20(28(19)12-13-4-2-1-3-5-13)22(31)27-23(32)29(21)14-6-7-14/h1-5,8-10,14,30H,6-7,11-12H2,(H,24,25)(H,27,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50556732

(CHEMBL4750435)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc3ccc(=O)oc3c2)c2cc(cnc12)-c1ccc(cc1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN1 (unknown origin) pre-incubated for 20 mins followed by fluorescence substrate addition and measured after 120 mins by DiFMUP assa... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50556734
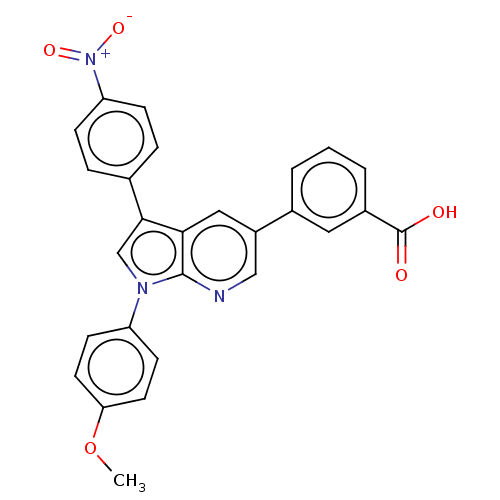
(CHEMBL4795725)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc(cc2)[N+]([O-])=O)c2cc(cnc12)-c1cccc(c1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 10 uM DiFMUP by DiFMUP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 1
(Homo sapiens (Human)) | BDBM357739

(AG-01-128/(I-202) | US10214530, Example 18)Show SMILES CCn1c2nc(Cc3nc4ccccc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O Show InChI InChI=1S/C22H20N6O2/c1-2-27-20-19(21(29)26-22(27)30)28(13-14-8-4-3-5-9-14)18(25-20)12-17-23-15-10-6-7-11-16(15)24-17/h3-11H,2,12-13H2,1H3,(H,23,24)(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357759

(KM-05-127/(I-16) | US10214530, Example 16)Show SMILES O=c1[nH]c(=O)c2n(Cc3ccccc3)c(Cc3nc4ccc(cc4[nH]3)C#N)nc2n1C1CC1 Show InChI InChI=1S/C24H19N7O2/c25-12-15-6-9-17-18(10-15)27-19(26-17)11-20-28-22-21(30(20)13-14-4-2-1-3-5-14)23(32)29-24(33)31(22)16-7-8-16/h1-6,9-10,16H,7-8,11,13H2,(H,26,27)(H,29,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357762

(KM430/(I-31) | US10214530, Example 37)Show SMILES Cn1c2nc(Sc3nc4cc(N)ccc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O Show InChI InChI=1S/C20H17N7O2S/c1-26-16-15(17(28)25-19(26)29)27(10-11-5-3-2-4-6-11)20(24-16)30-18-22-13-8-7-12(21)9-14(13)23-18/h2-9H,10,21H2,1H3,(H,22,23)(H,25,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357769

(KM446/(I-35) | US10214530, Example 41)Show SMILES Cc1ccc2[nH]c(Sc3nc4n(C)c(=O)[nH]c(=O)c4n3Cc3cncs3)nc2c1 Show InChI InChI=1S/C18H15N7O2S2/c1-9-3-4-11-12(5-9)21-16(20-11)29-18-22-14-13(15(26)23-17(27)24(14)2)25(18)7-10-6-19-8-28-10/h3-6,8H,7H2,1-2H3,(H,20,21)(H,23,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50556730
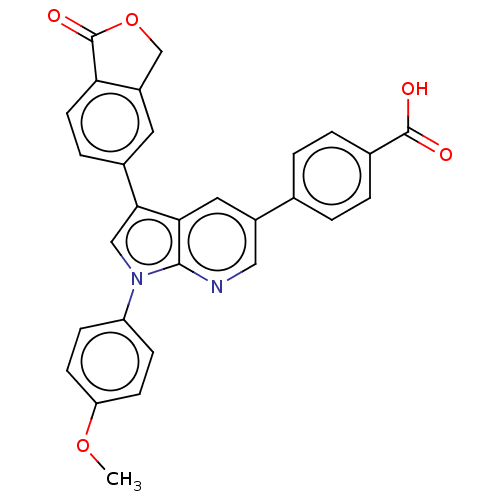
(CHEMBL4777804)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc3C(=O)OCc3c2)c2cc(cnc12)-c1ccc(cc1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SHP2 (262 to 532 amino acids) (unknown origin) incubated for 1 hr using 20 uM DiFMUP by DiFMUP assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 1
(Homo sapiens (Human)) | BDBM357740

(KM-05-139/(I-23) | US10214530, Example 23)Show SMILES O=c1[nH]c(=O)c2n(Cc3ccccc3)c(Cc3nc4cc5OCOc5cc4[nH]3)nc2n1C1CC1 Show InChI InChI=1S/C24H20N6O4/c31-23-21-22(30(14-6-7-14)24(32)28-23)27-20(29(21)11-13-4-2-1-3-5-13)10-19-25-15-8-17-18(34-12-33-17)9-16(15)26-19/h1-5,8-9,14H,6-7,10-12H2,(H,25,26)(H,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |
Tryptophan 5-hydroxylase 2
(Homo sapiens (Human)) | BDBM357761
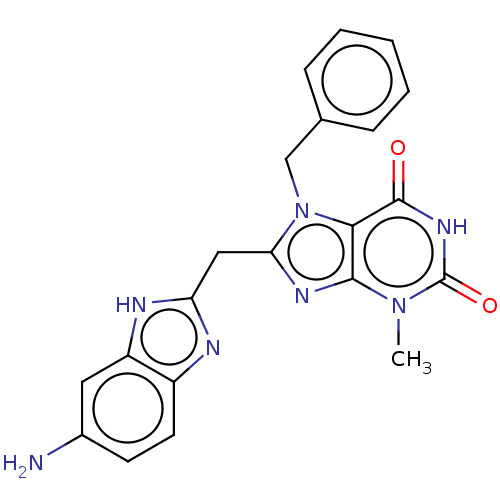
(KM-05-55 Fr10-12/(l-6) | US10214530, Example 6)Show SMILES Cn1c2nc(Cc3nc4ccc(N)cc4[nH]3)n(Cc3ccccc3)c2c(=O)[nH]c1=O Show InChI InChI=1S/C21H19N7O2/c1-27-19-18(20(29)26-21(27)30)28(11-12-5-3-2-4-6-12)17(25-19)10-16-23-14-8-7-13(22)9-15(14)24-16/h2-9H,10-11,22H2,1H3,(H,23,24)(H,26,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00598
BindingDB Entry DOI: 10.7270/Q2G164VF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data