

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119782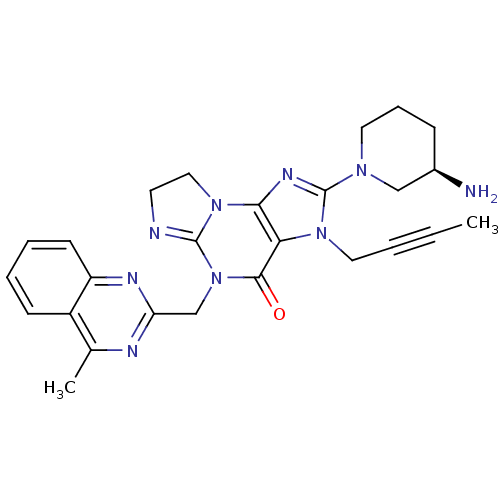 (US8691832, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119786 (US8691832, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119788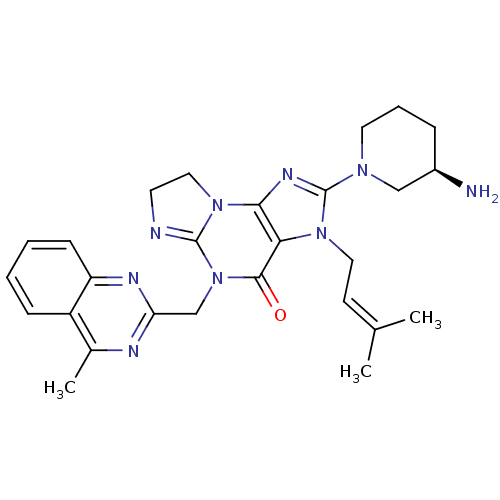 (US8691832, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM60417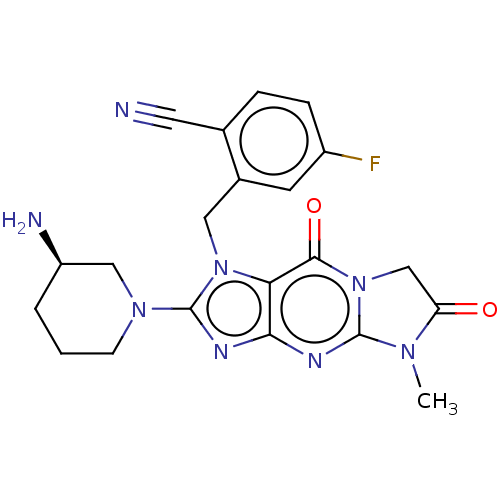 (US9051329, Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499796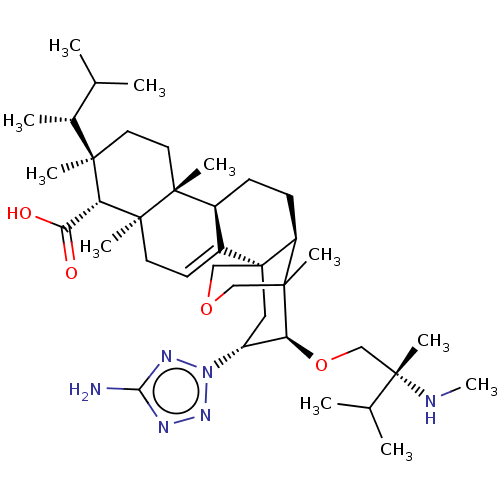 (CHEMBL3742110) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.99 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499806 (CHEMBL3740011) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50534235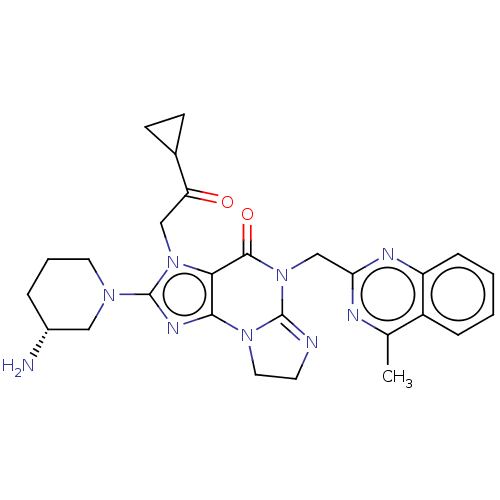 (CHEMBL4464819) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499797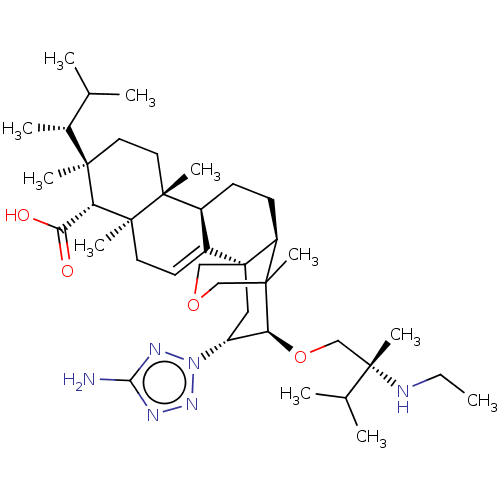 (CHEMBL3742052) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM16285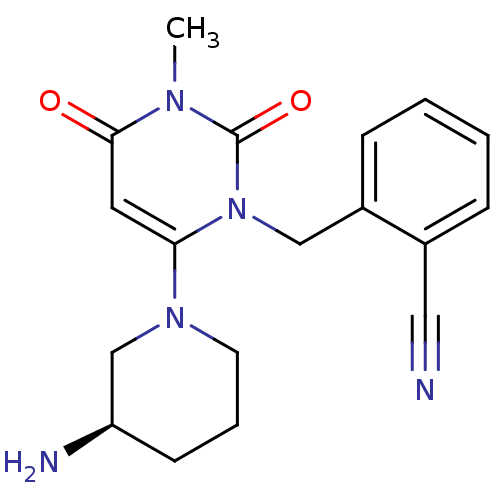 (2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119791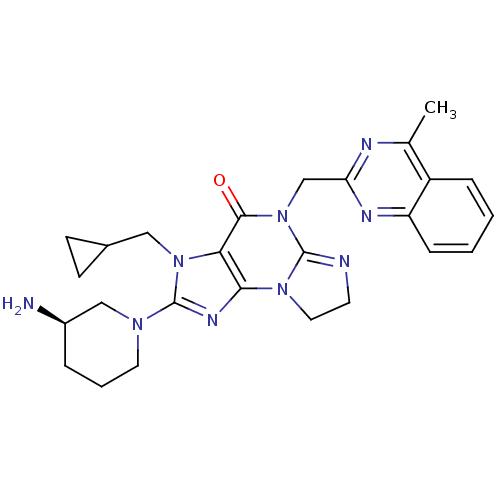 (US8691832, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119796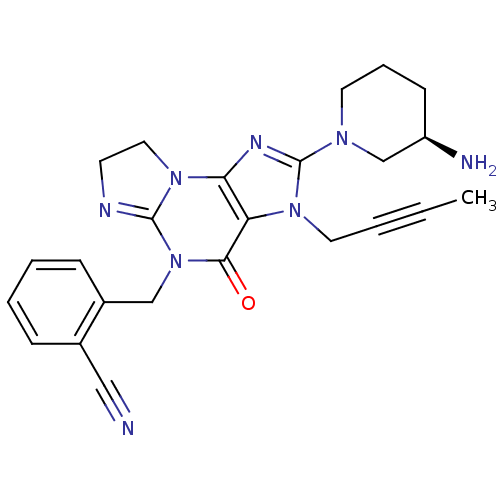 (US8691832, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499793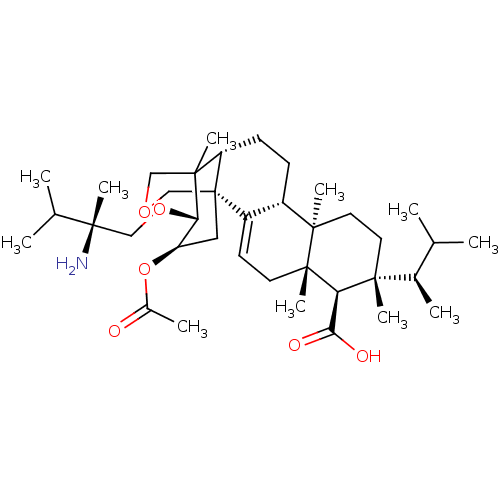 (CHEMBL3739474) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50534237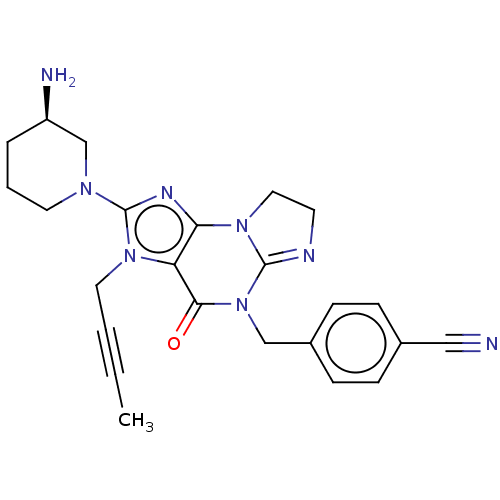 (CHEMBL4568895) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50534238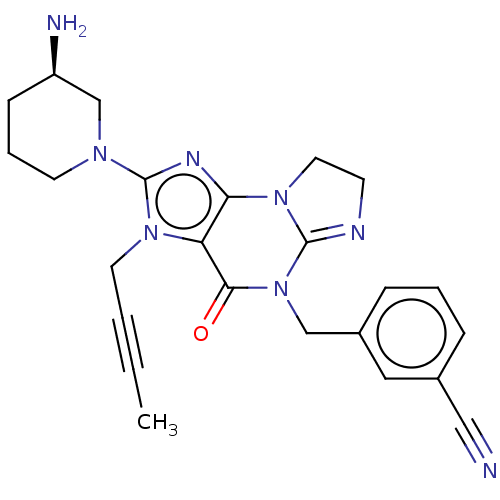 (CHEMBL4516443) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM162377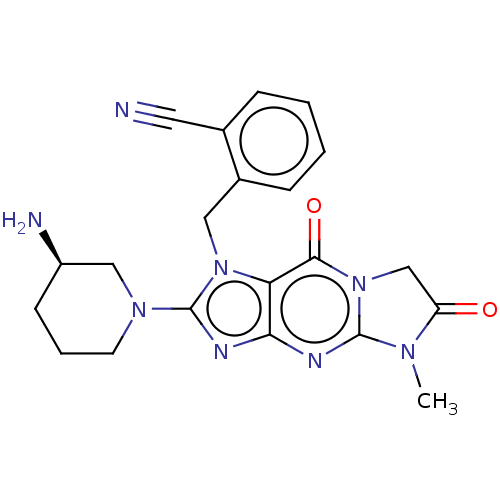 (US9051329, Example 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM162383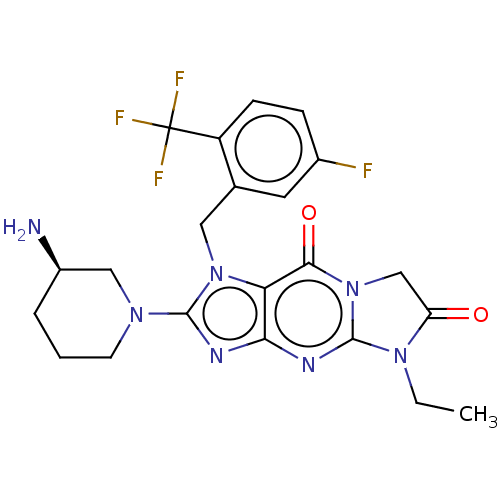 (US9051329, Example 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499808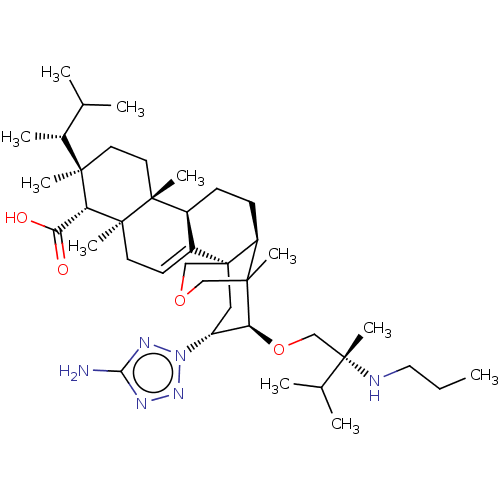 (CHEMBL3741296) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499809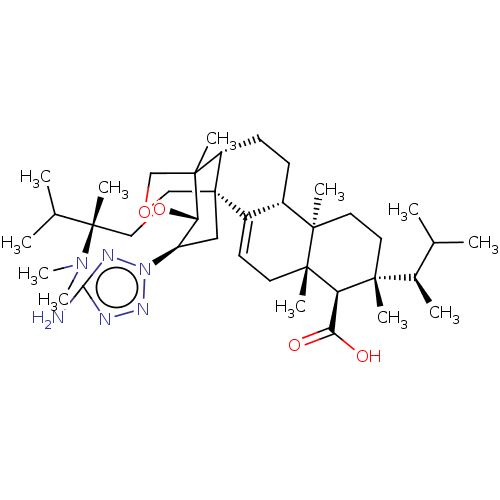 (CHEMBL3741220) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50177780 (CHEMBL3813856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119798 (US8691832, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499807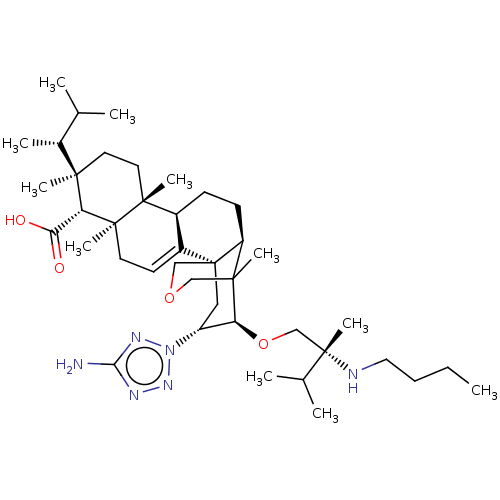 (CHEMBL3740280) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119789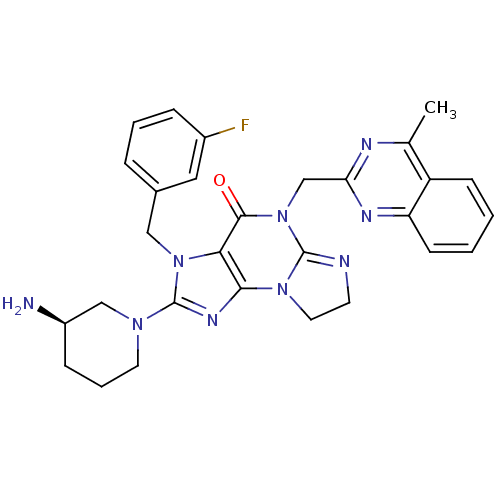 (US8691832, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499799 (CHEMBL3741985) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499794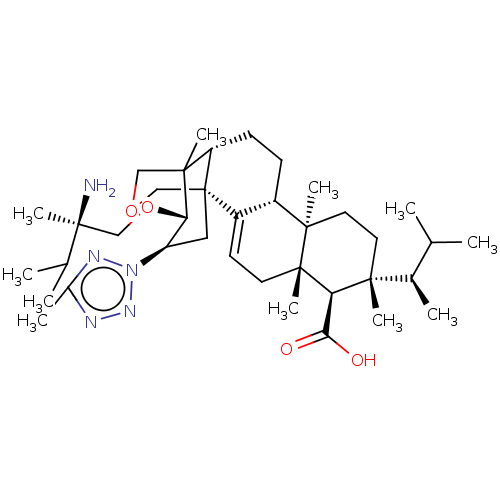 (CHEMBL3741163) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM162376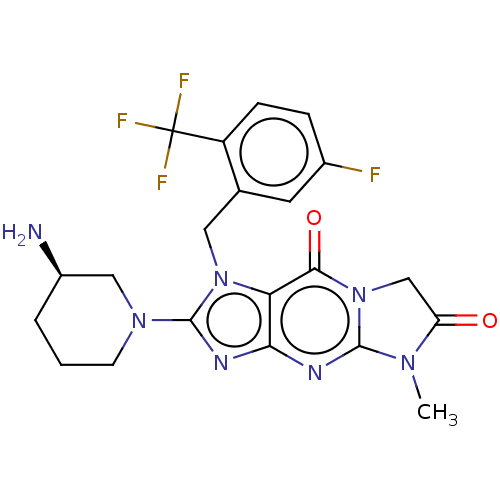 (US9051329, Example 1 | US9051329, Example 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50534236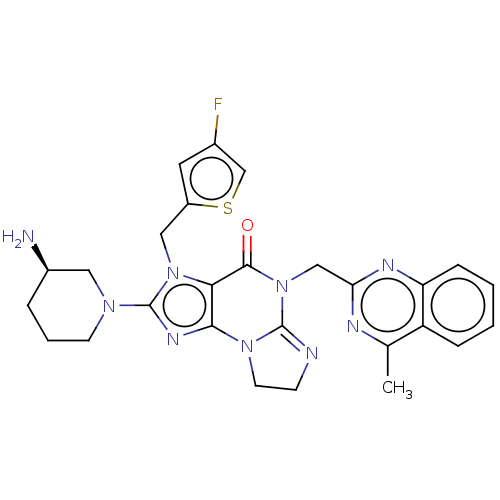 (CHEMBL4563476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119799 (US8691832, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119790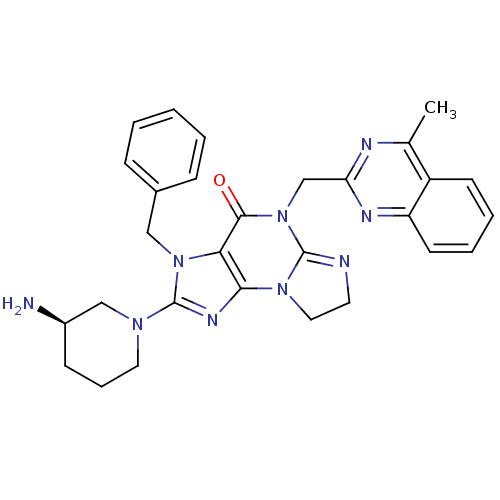 (US8691832, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119792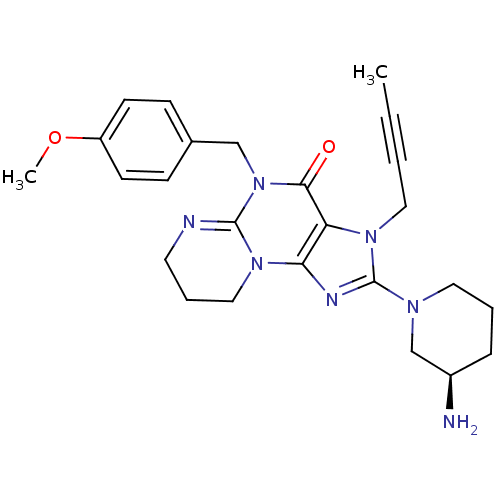 (US8691832, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119794 (US8691832, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119784 (US8691832, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499811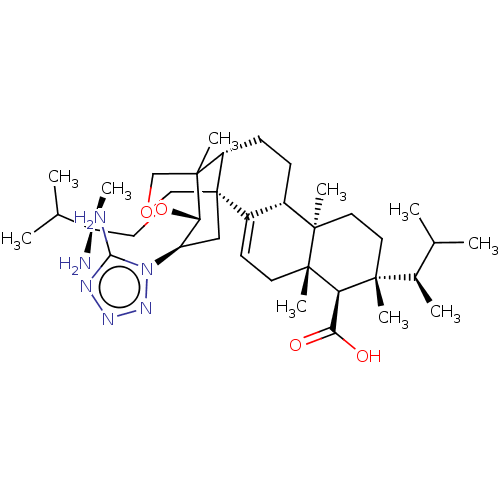 (CHEMBL3742174) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-protein thioesterase 1 (Rattus norvegicus) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FAP (unknown origin) preincubated for 20 mins followed by Nle-Pro-AMC addition measured for 40 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499805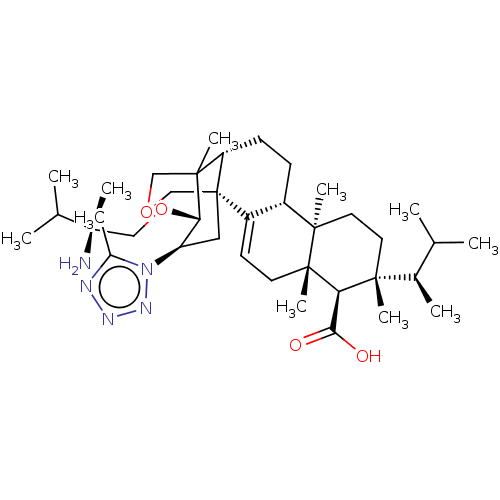 (CHEMBL3741171) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499792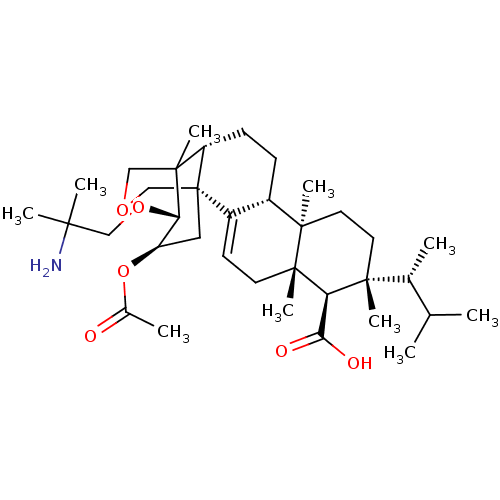 (CHEMBL3739756) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499810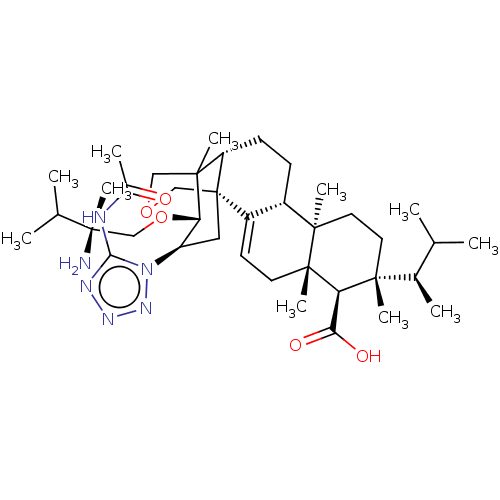 (CHEMBL3741086) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 81.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499804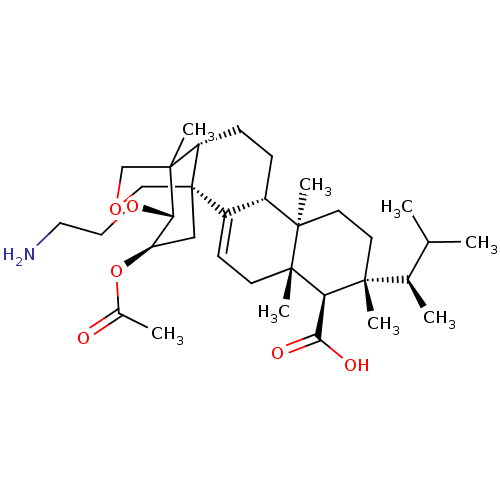 (CHEMBL3740861) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 81.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM119800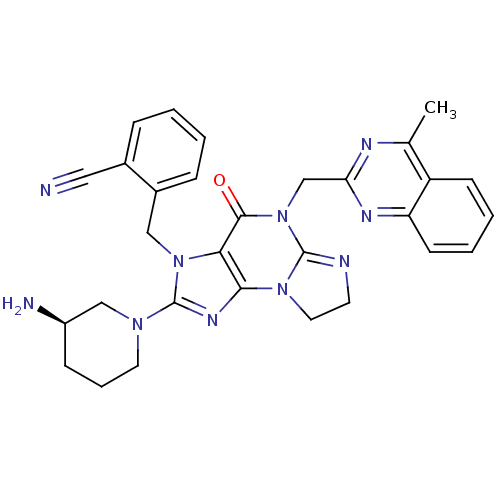 (US8691832, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499795 (CHEMBL3740165) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 86.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499787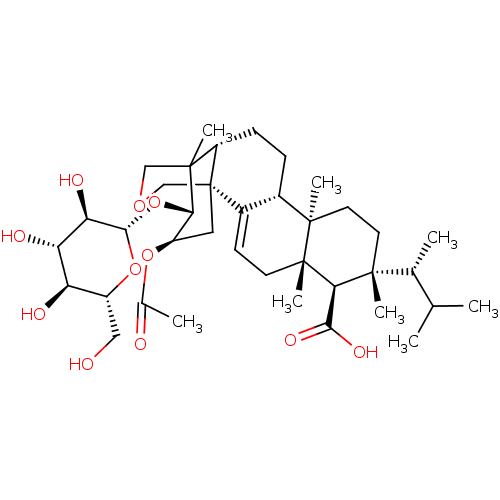 (CHEMBL3740212) | UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 86.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-protein thioesterase 1 (Rattus norvegicus) | BDBM50534235 (CHEMBL4464819) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FAP (unknown origin) using Nle-Pro-AMC as substrate preincubated for 20 mins followed by substrate addition measured after 40 mins by f... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM162380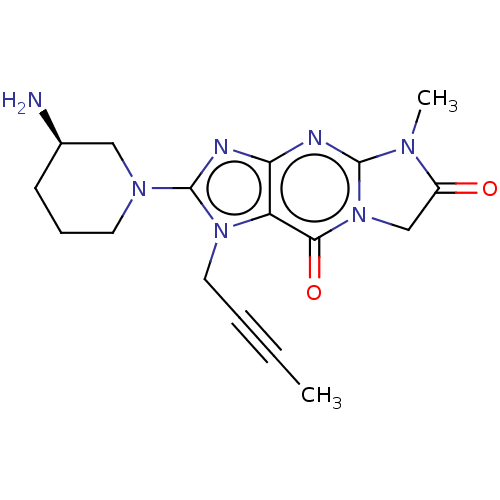 (US9051329, Example 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM162378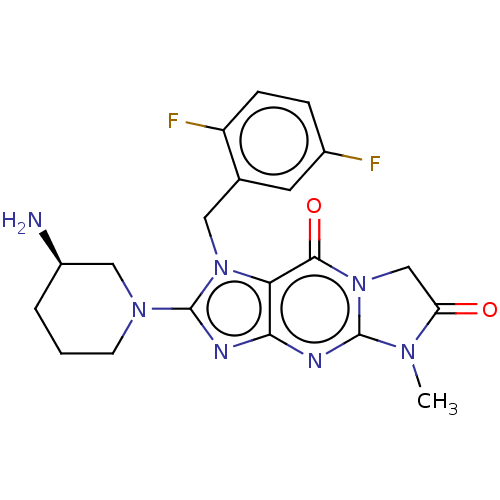 (US9051329, Example 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-protein thioesterase 1 (Rattus norvegicus) | BDBM119786 (US8691832, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FAP (unknown origin) using Nle-Pro-AMC as substrate preincubated for 20 mins followed by substrate addition measured after 40 mins by f... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-D-glucan synthase catalytic subunit (Candida albicans) | BDBM50499801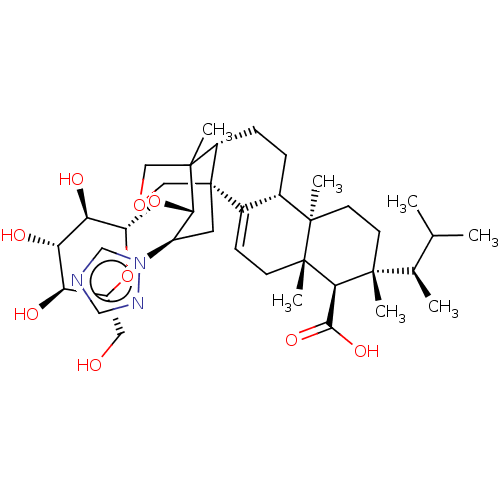 (CHEMBL3740840) | UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 341 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of UDP-[3H]glucose from beta-1,3-glucan synthase in Candida albicans MY1055 microsomal membranes incubated for 2 hrs | Bioorg Med Chem Lett 25: 5813-8 (2015) Article DOI: 10.1016/j.bmcl.2015.10.011 BindingDB Entry DOI: 10.7270/Q2DF6V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-protein thioesterase 1 (Rattus norvegicus) | BDBM119782 (US8691832, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 367 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FAP (unknown origin) using Nle-Pro-AMC as substrate preincubated for 20 mins followed by substrate addition measured after 40 mins by f... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50534233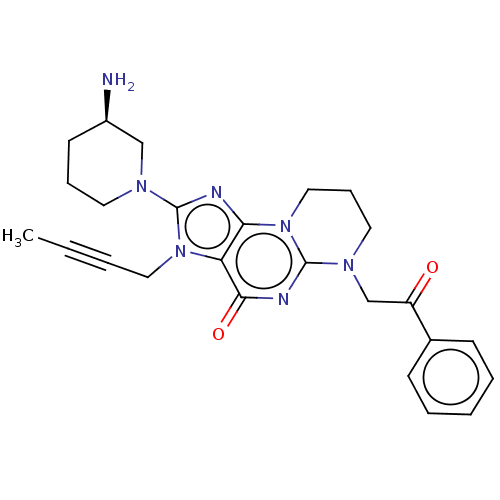 (CHEMBL4591675) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 421 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50534239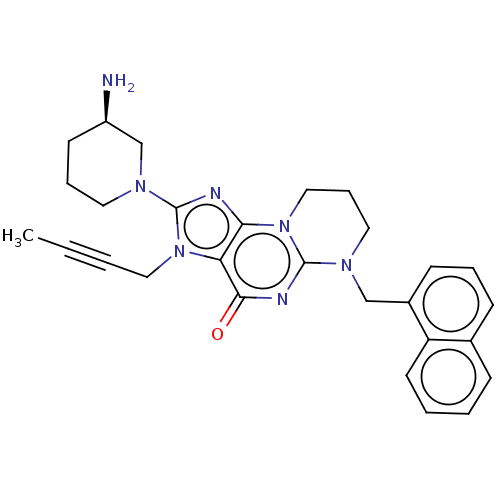 (CHEMBL4537708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 483 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50534240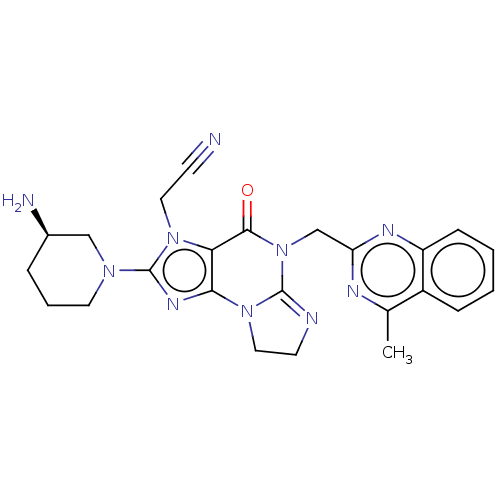 (CHEMBL4443070) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 538 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) using Gly-Pro-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 50 mins by ... | Bioorg Med Chem 24: 5534-5545 (2016) Article DOI: 10.1016/j.bmc.2016.09.007 BindingDB Entry DOI: 10.7270/Q2GX4G2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 517 total ) | Next | Last >> |