

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106090 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((4-methoxy-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106091 ((S)-3-Benzo[1,3]dioxol-5-yl-3-((S)-4-methyl-2-{2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106083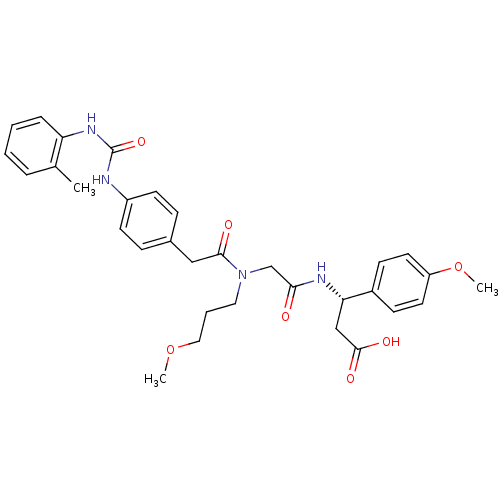 ((S)-3-(4-Methoxy-phenyl)-3-[2-((3-methoxy-propyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106086 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methoxy-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106085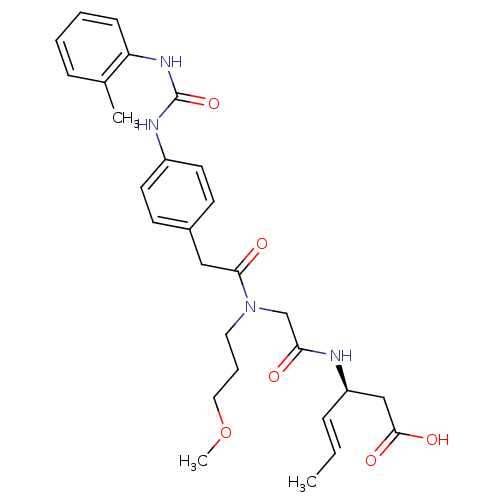 ((E)-(S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by mouse Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106085 ((E)-(S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to fibronectin by Very late antigen 4 (VLA-4) | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106082 ((S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-urei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106086 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methoxy-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to fibronectin by Very late antigen 5 (VLA-5) | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106082 ((S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-urei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to fibronectin by Very late antigen 4 (VLA-4) | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106085 ((E)-(S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106098 ((R)-3-((S)-4-Methyl-2-{2-[4-(3-o-tolyl-ureido)-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106093 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methyl-butyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106085 ((E)-(S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by rat Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106082 ((S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-urei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by mouse Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106086 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methoxy-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by rat Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106082 ((S)-3-[2-((3-Methoxy-propyl)-{2-[4-(3-o-tolyl-urei...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by rat Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106086 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methoxy-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by mouse Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106083 ((S)-3-(4-Methoxy-phenyl)-3-[2-((3-methoxy-propyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by rat Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106099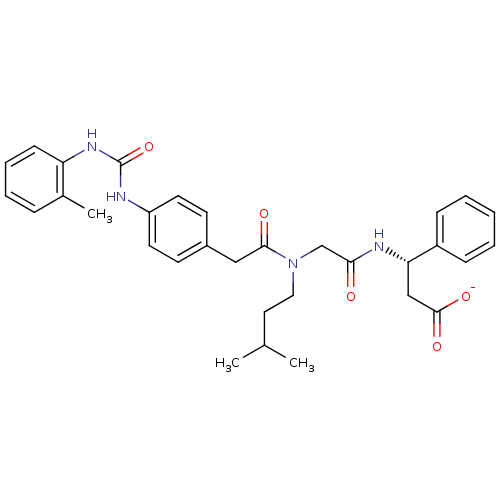 (CHEMBL321739 | Lithium; (S)-3-[2-((3-methyl-butyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106083 ((S)-3-(4-Methoxy-phenyl)-3-[2-((3-methoxy-propyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to fibronectin by Very late antigen 5 (VLA-5) | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106102 ((E)-(S)-3-[2-((3-Methyl-butyl)-{2-[4-(3-o-tolyl-ur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106083 ((S)-3-(4-Methoxy-phenyl)-3-[2-((3-methoxy-propyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by mouse Very late antigen 4 (VLA-4) integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106097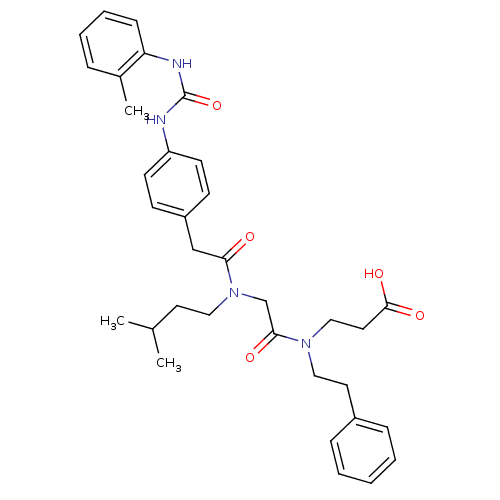 (3-{[2-((3-Methyl-butyl)-{2-[4-(3-o-tolyl-ureido)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A (Escherichia coli (strain K12)) | BDBM50457571 (CHEMBL4218301) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of His tagged Escherichia coli ATCC 25922 DNA gyrase A SD-LY mutant expressed in Escherichia coli BL21 (DE3) pLysS cells assessed as reduc... | J Med Chem 61: 4456-4475 (2018) Article DOI: 10.1021/acs.jmedchem.8b00114 BindingDB Entry DOI: 10.7270/Q2H997TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50520671 (CHEMBL4459971) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of human ERG at 37 degC | J Med Chem 61: 6501-6517 (2018) Article DOI: 10.1021/acs.jmedchem.8b00741 BindingDB Entry DOI: 10.7270/Q24171F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A (Escherichia coli (strain K12)) | BDBM21690 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of His tagged Escherichia coli ATCC 25922 DNA gyrase A SD-LY mutant expressed in Escherichia coli BL21 (DE3) pLysS cells assessed as reduc... | J Med Chem 61: 4456-4475 (2018) Article DOI: 10.1021/acs.jmedchem.8b00114 BindingDB Entry DOI: 10.7270/Q2H997TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106087 (1,3-Diphenyl-urea derivative | CHEMBL98307) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106094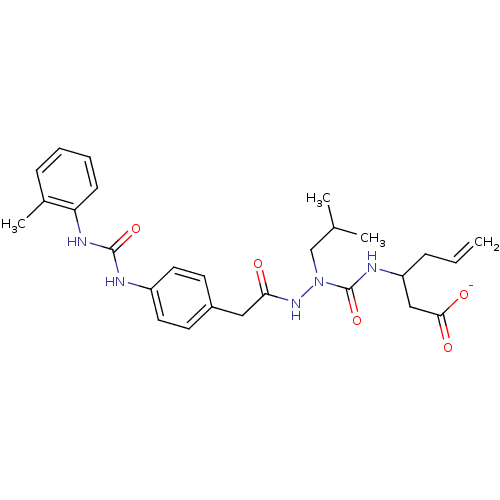 (1,3-Diphenyl-urea derivative | CHEMBL98793) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-7 (Homo sapiens (Human)) | BDBM50106083 ((S)-3-(4-Methoxy-phenyl)-3-[2-((3-methoxy-propyl)-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by alpha4-beta7 integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106088 (CHEMBL99888 | Lithium; (R)-((S)-5-methyl-3-{2-[4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106095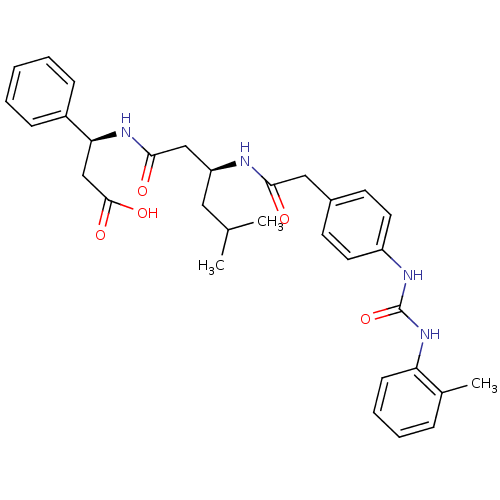 ((S)-3-((S)-5-Methyl-3-{2-[4-(3-o-tolyl-ureido)-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50448109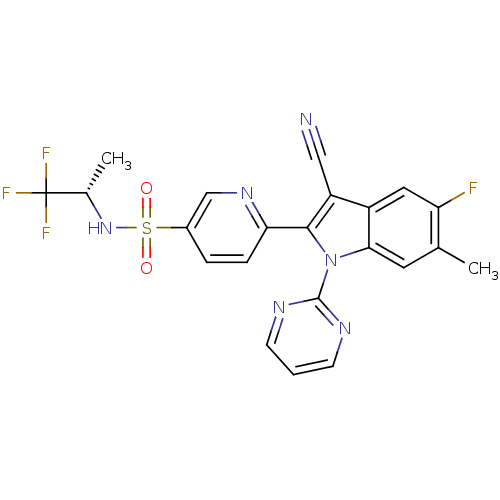 (CHEMBL3121811) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 57: 2121-35 (2014) Article DOI: 10.1021/jm401621g BindingDB Entry DOI: 10.7270/Q2RR20RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50448108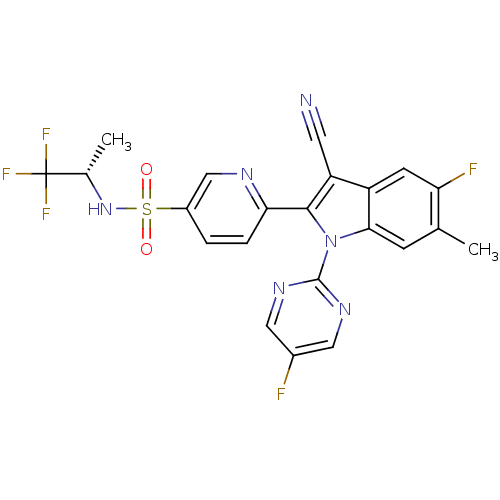 (CHEMBL3121694) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 57: 2121-35 (2014) Article DOI: 10.1021/jm401621g BindingDB Entry DOI: 10.7270/Q2RR20RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50448107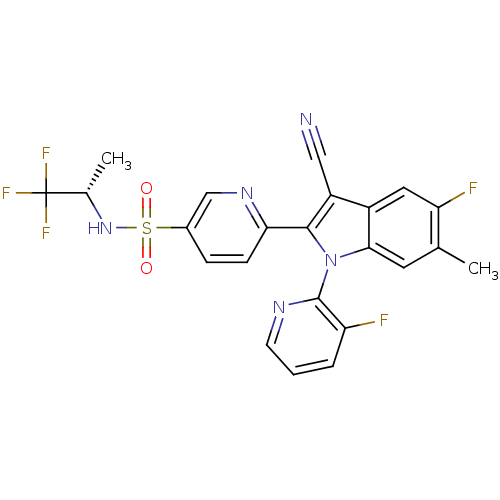 (CHEMBL3121798) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 57: 2121-35 (2014) Article DOI: 10.1021/jm401621g BindingDB Entry DOI: 10.7270/Q2RR20RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50448106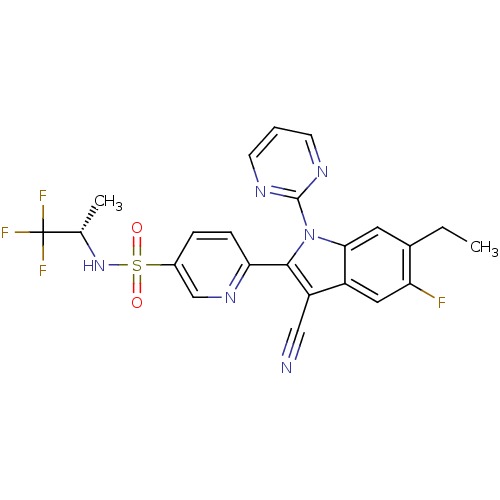 (CHEMBL3120505) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 57: 2121-35 (2014) Article DOI: 10.1021/jm401621g BindingDB Entry DOI: 10.7270/Q2RR20RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50448105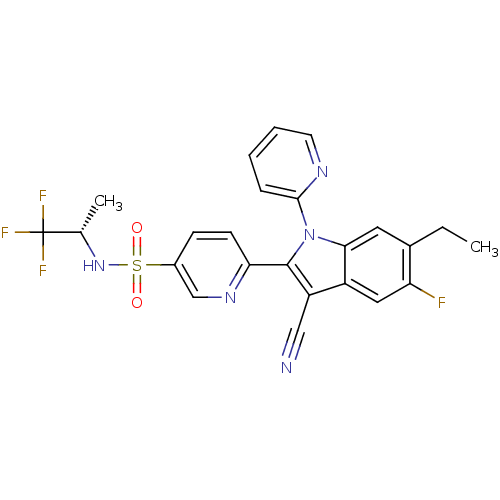 (CHEMBL3121800) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 57: 2121-35 (2014) Article DOI: 10.1021/jm401621g BindingDB Entry DOI: 10.7270/Q2RR20RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50448104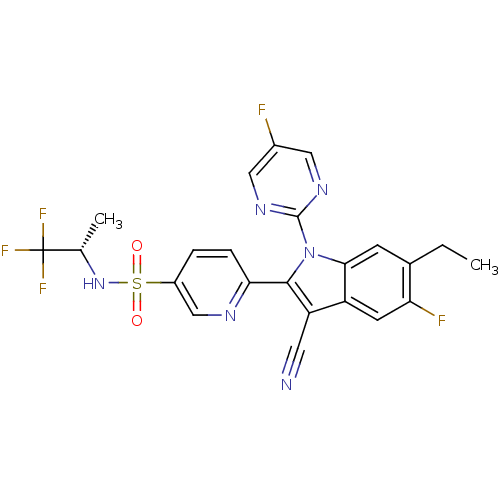 (CHEMBL3121804) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 57: 2121-35 (2014) Article DOI: 10.1021/jm401621g BindingDB Entry DOI: 10.7270/Q2RR20RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50448109 (CHEMBL3121811) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | J Med Chem 57: 2121-35 (2014) Article DOI: 10.1021/jm401621g BindingDB Entry DOI: 10.7270/Q2RR20RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50448108 (CHEMBL3121694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | J Med Chem 57: 2121-35 (2014) Article DOI: 10.1021/jm401621g BindingDB Entry DOI: 10.7270/Q2RR20RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50448107 (CHEMBL3121798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | J Med Chem 57: 2121-35 (2014) Article DOI: 10.1021/jm401621g BindingDB Entry DOI: 10.7270/Q2RR20RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50448106 (CHEMBL3120505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | J Med Chem 57: 2121-35 (2014) Article DOI: 10.1021/jm401621g BindingDB Entry DOI: 10.7270/Q2RR20RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50448104 (CHEMBL3121804) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | J Med Chem 57: 2121-35 (2014) Article DOI: 10.1021/jm401621g BindingDB Entry DOI: 10.7270/Q2RR20RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50448105 (CHEMBL3121800) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PTC Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | J Med Chem 57: 2121-35 (2014) Article DOI: 10.1021/jm401621g BindingDB Entry DOI: 10.7270/Q2RR20RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106084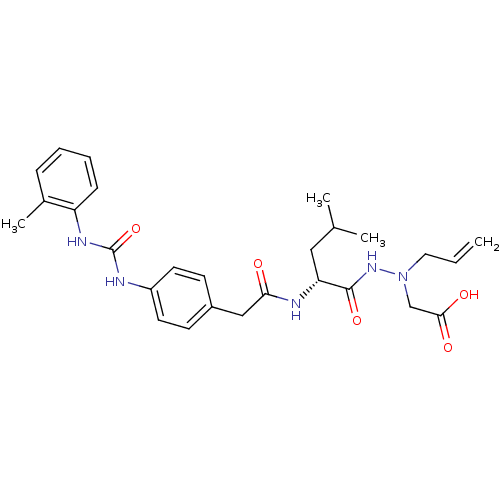 (CHEMBL103417 | [N-Allyl-N'-((R)-4-methyl-2-{2-[4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106096 (3-[((R)-4-Methyl-2-{2-[4-(3-o-tolyl-ureido)-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50266625 (CHEMBL4081789) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 60: 4444-4457 (2017) Article DOI: 10.1021/acs.jmedchem.7b00406 BindingDB Entry DOI: 10.7270/Q2N300FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50266625 (CHEMBL4081789) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 60: 4444-4457 (2017) Article DOI: 10.1021/acs.jmedchem.7b00406 BindingDB Entry DOI: 10.7270/Q2N300FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50266625 (CHEMBL4081789) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 60: 4444-4457 (2017) Article DOI: 10.1021/acs.jmedchem.7b00406 BindingDB Entry DOI: 10.7270/Q2N300FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50106089 ((S)-3-(4-Methoxy-phenyl)-3-[2-((3-methoxy-propyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro Very late antigen 4 cell adhesion inhibitory activity measured by binding of fluorescently labeled Ramos cells to immobilized VCAM-1. | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-7 (Homo sapiens (Human)) | BDBM50106086 ((S)-3-(3,4-Dimethoxy-phenyl)-3-[2-((3-methoxy-prop...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of fluorescent Ramos cell adhesion to VCAM-1 by alpha4-beta7 integrin | Bioorg Med Chem Lett 11: 2955-8 (2001) BindingDB Entry DOI: 10.7270/Q20001CZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |