

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50400024 (CHEMBL2177719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50400020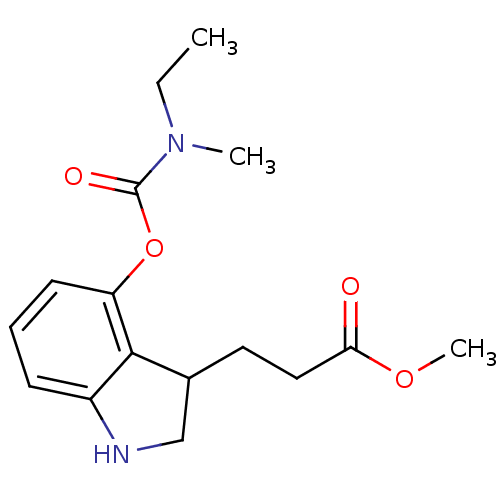 (CHEMBL2181475) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50013328 (CHEMBL3263347) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50400024 (CHEMBL2177719) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50400027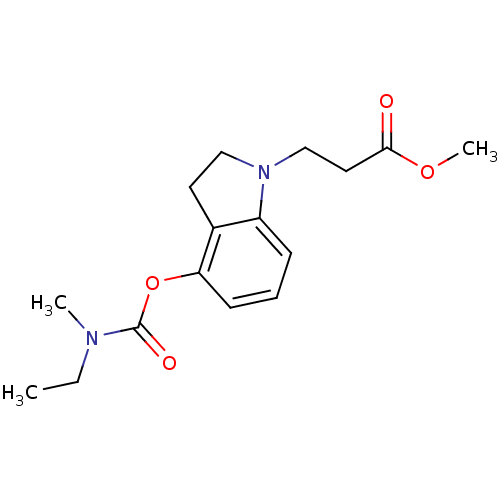 (CHEMBL2177716) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50013334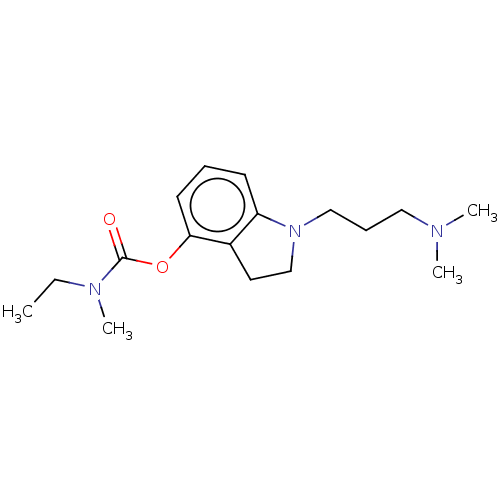 (CHEMBL3263351) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50013332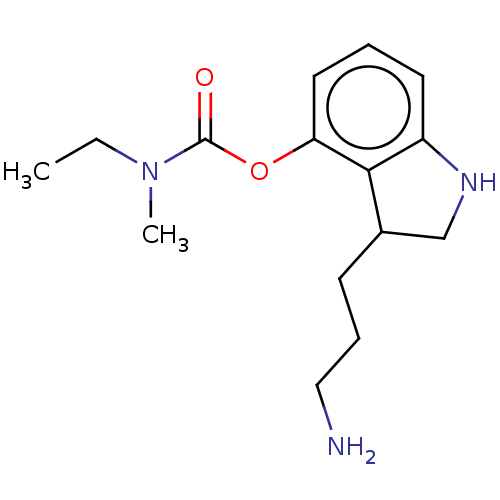 (CHEMBL3263349) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50013328 (CHEMBL3263347) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50013332 (CHEMBL3263349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50013331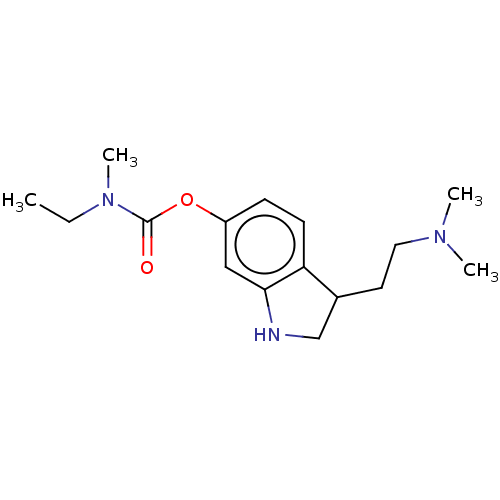 (CHEMBL3263348) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16189 (4-(3-Iodophenyl)-3-oxobutanenitrile | beta-ketonit...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50013334 (CHEMBL3263351) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50400009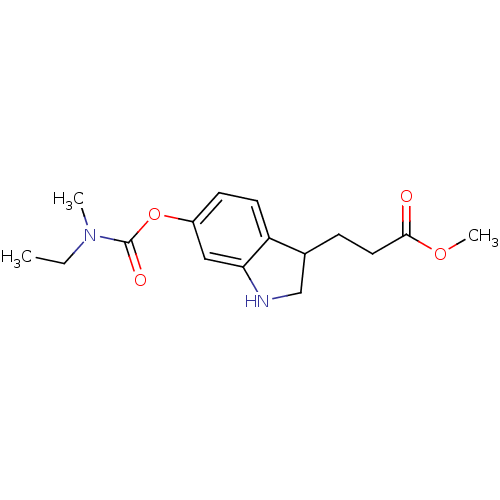 (CHEMBL2177708) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16185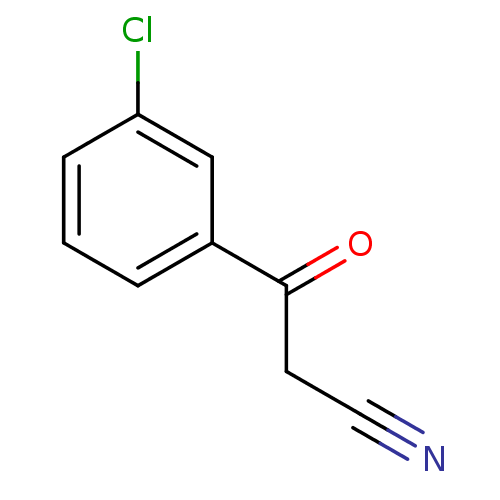 (3-(3-Chlorophenyl)-3-oxopropanenitrile | beta-keto...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50013382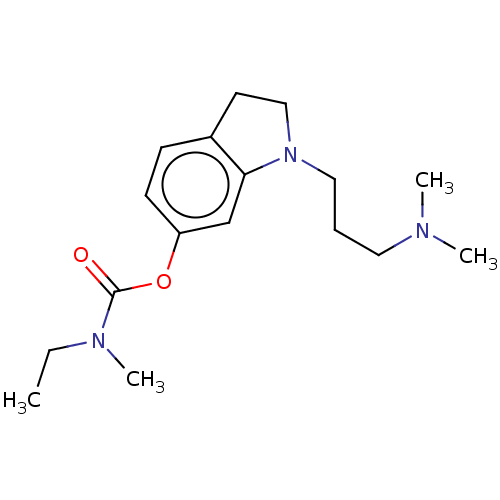 (CHEMBL3263352) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50013333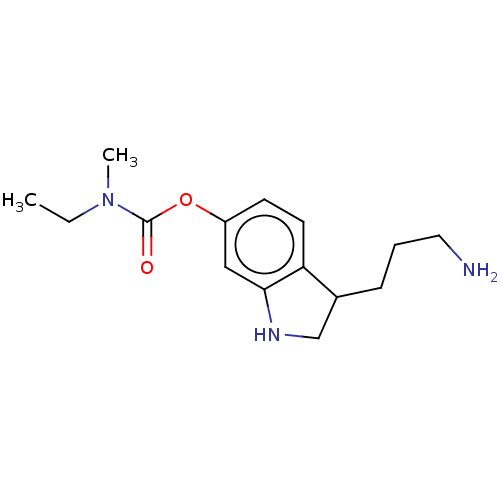 (CHEMBL3263350) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50400027 (CHEMBL2177716) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50400025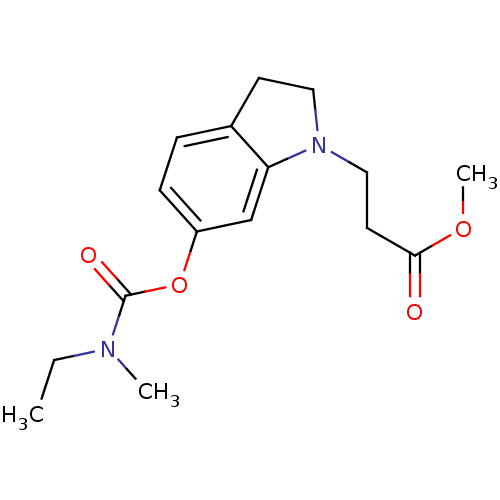 (CHEMBL2177718) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of BuChE in equine serum using butylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50400020 (CHEMBL2181475) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [600-1159,L699I,K702N,C879S] (Human immunodeficiency virus type 1) | BDBM16189 (4-(3-Iodophenyl)-3-oxobutanenitrile | beta-ketonit...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16187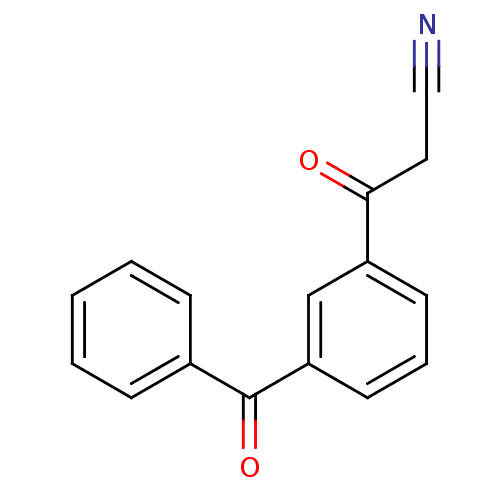 (3-(3-benzoylphenyl)-3-oxopropanenitrile | 3-Benzoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1159,Y780C]/Imidazoleglycerol-phosphate dehydratase [600-1029,Y780C] (Human immunodeficiency virus type 1) | BDBM16189 (4-(3-Iodophenyl)-3-oxobutanenitrile | beta-ketonit...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16196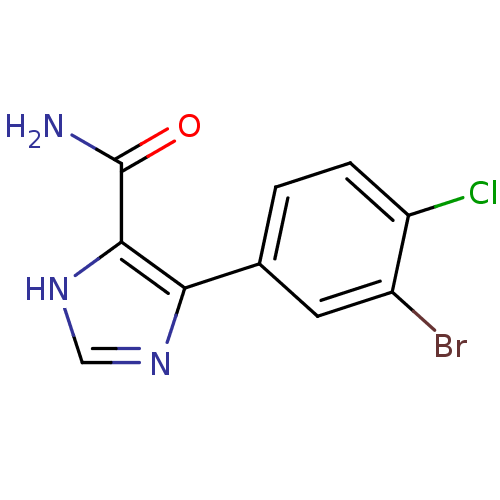 (4-(3-Bromo-4-chlorophenyl)-1H-imidazole-5-carboxam...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16208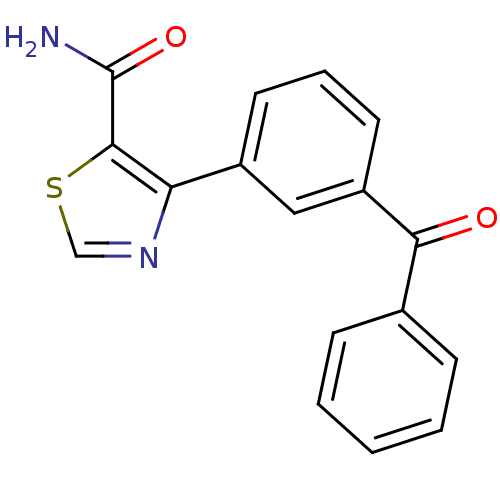 (4-(3-Benzoylphenyl)thiazole-5-carboxamide | 4-(3-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [600-1159,L699I,K702N,C879S] (Human immunodeficiency virus type 1) | BDBM16196 (4-(3-Bromo-4-chlorophenyl)-1H-imidazole-5-carboxam...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50013331 (CHEMBL3263348) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1159,Y780C]/Imidazoleglycerol-phosphate dehydratase [600-1029,Y780C] (Human immunodeficiency virus type 1) | BDBM16196 (4-(3-Bromo-4-chlorophenyl)-1H-imidazole-5-carboxam...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16191 (3-(3-Iodophenyl)-3-oxopropanamide | beta-ketoamide...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.96E+4 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16188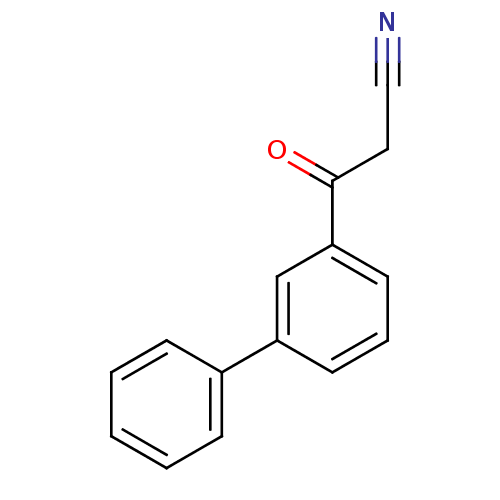 (3-Phenyl-3-oxopropanenitrile | 3-oxo-3-(3-phenylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.34E+4 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50013333 (CHEMBL3263350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16203 (2-Amino-4-(3-benzoylphenyl)thiazole-5-carboxamide ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50013382 (CHEMBL3263352) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16201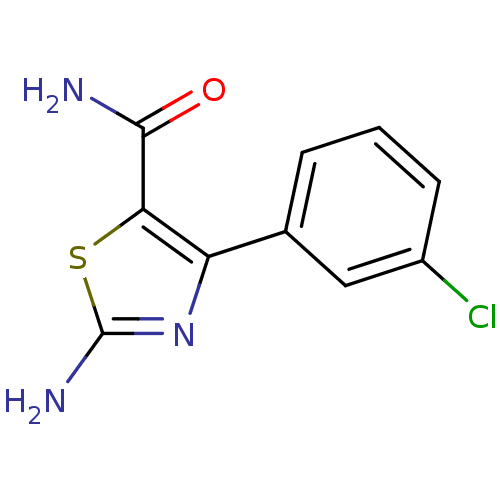 (2-Amino-4-(3-chlorophenyl)thiazole-5-carboxamide |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50400009 (CHEMBL2177708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem Lett 24: 2283-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.081 BindingDB Entry DOI: 10.7270/Q21V5GHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16204 (2-Amino-4-(3-phenylphenyl)thiazole-5-carboxamide |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16190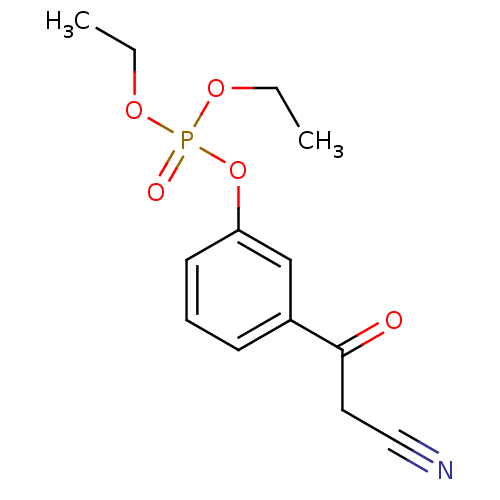 (3-(2-Cyanoacetyl)phenyl Diethyl Phosphate | [3-(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.43E+4 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [600-1159,L699I,K702N,C879S] (Human immunodeficiency virus type 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 7.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16195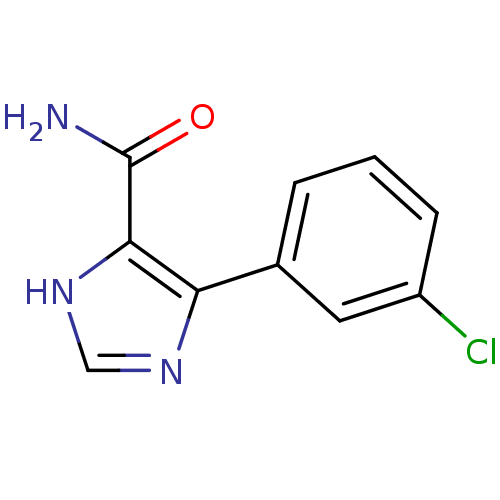 (4-(3-Chlorophenyl)-1H-imidazole-5-carboxamide | Im...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.98E+4 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16205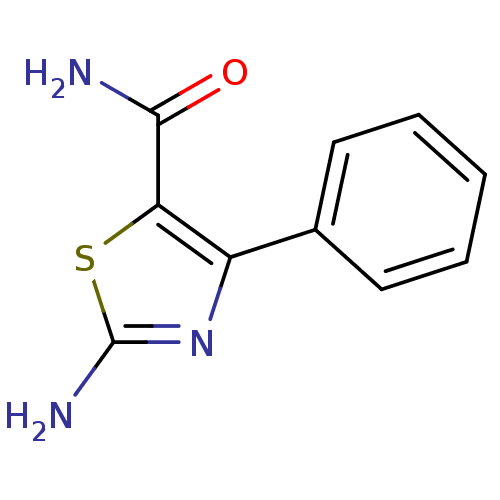 (2-Amino-4-phenylthiazole-5-carboxamide | 2-amino-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16207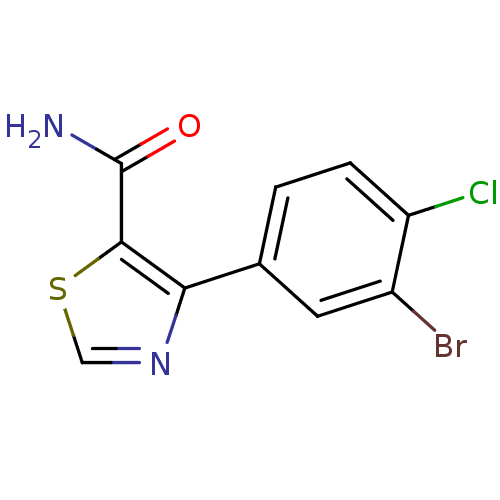 (4-(3-Bromo-4-chlorophenyl)thiazole-5-carboxamide |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1159,Y780C]/Imidazoleglycerol-phosphate dehydratase [600-1029,Y780C] (Human immunodeficiency virus type 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16202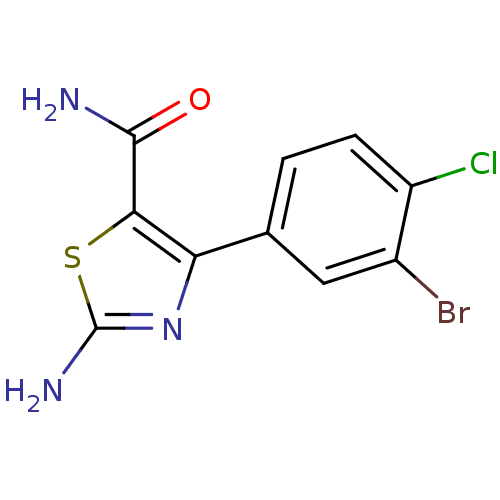 (2-Amino-4-(3-bromo-4-chlorophenyl)thiazole-5-carbo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16200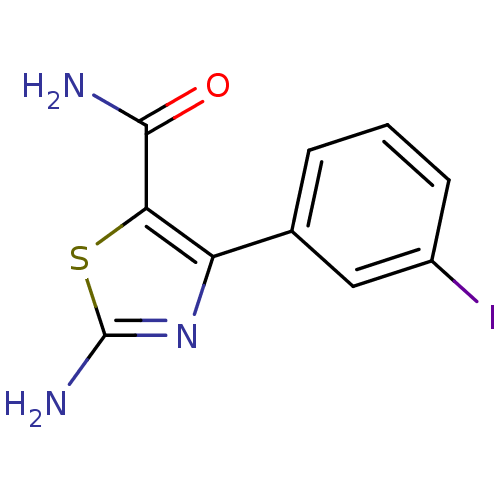 (2-Amino-4-(3-iodophenyl)thiazole-5-carboxamide | 2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16210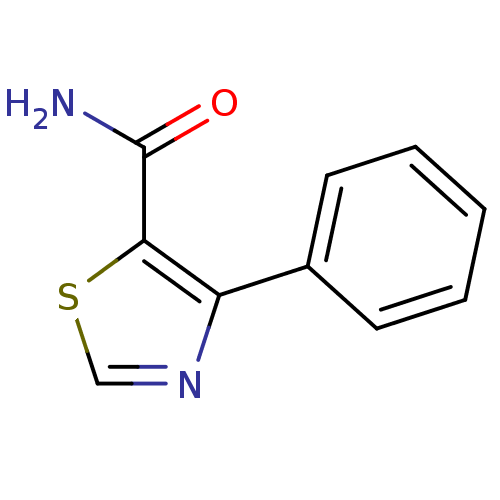 (4-Phenylthiazole-5-carboxamide | 4-phenyl-1,3-thia...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16199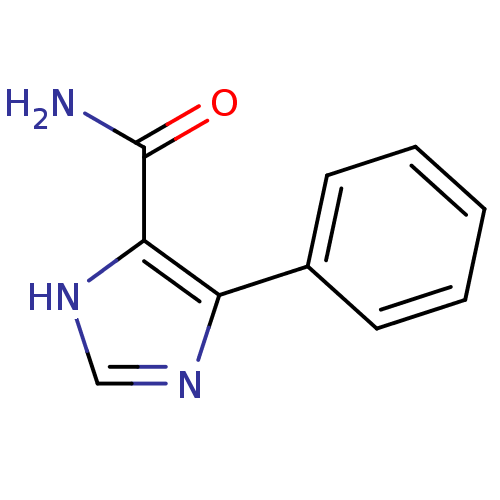 (4-Phenyl-1H-imidazole-5-carboxamide | Imidazole 8g) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16198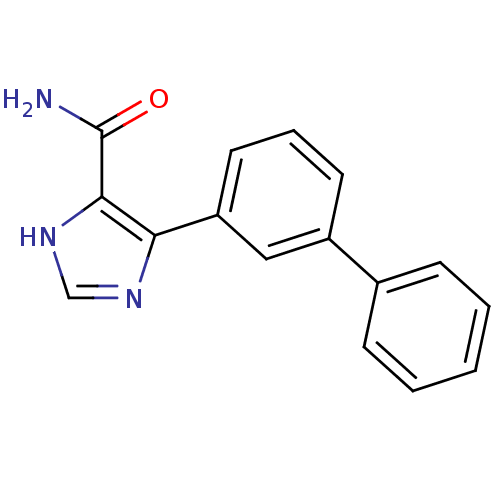 (4-(3-Phenylphenyl)-1H-imidazole-5-carboxamide | Im...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16197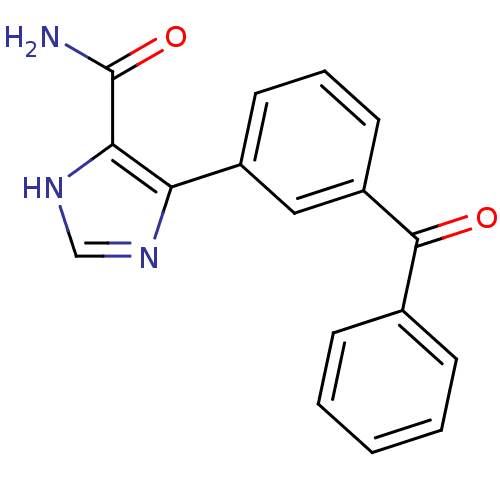 (4-(3-Benzoylphenyl)-1H-imidazole-5-carboxamide | I...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.12E+5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16194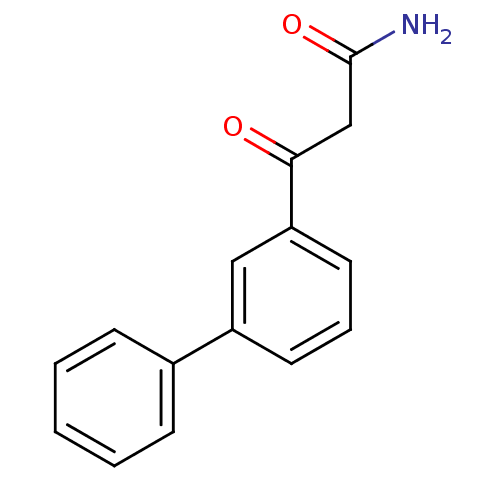 (3-Oxo-3-(3-phenylphenyl)propanamide | beta-ketoami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.12E+5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1029,C879S]/[600-1159,C879S] (Human immunodeficiency virus type 1) | BDBM16206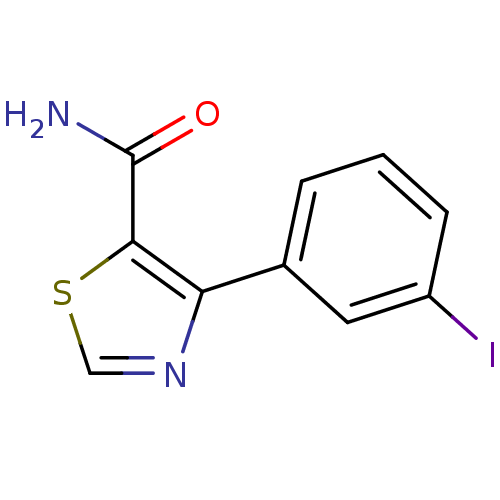 (4-(3-Iodophenyl)thiazole-5-carboxamide | 4-(3-iodo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar Ilan University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 50: 2370-84 (2007) Article DOI: 10.1021/jm0613121 BindingDB Entry DOI: 10.7270/Q2VM49J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |