

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50256789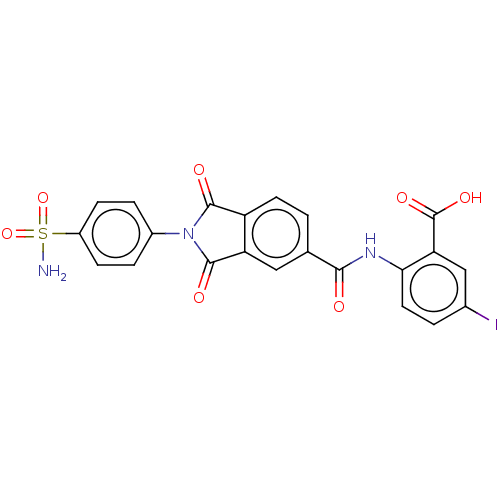 (CHEMBL4082361) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50256787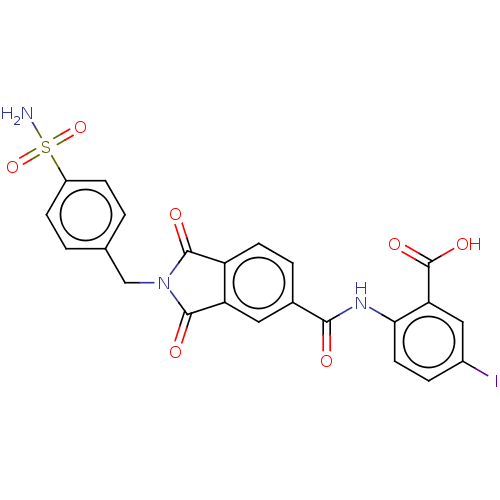 (CHEMBL4105131) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50256758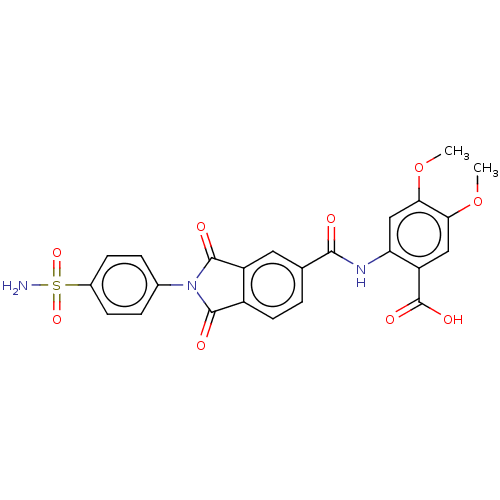 (CHEMBL4059883) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50256787 (CHEMBL4105131) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50256757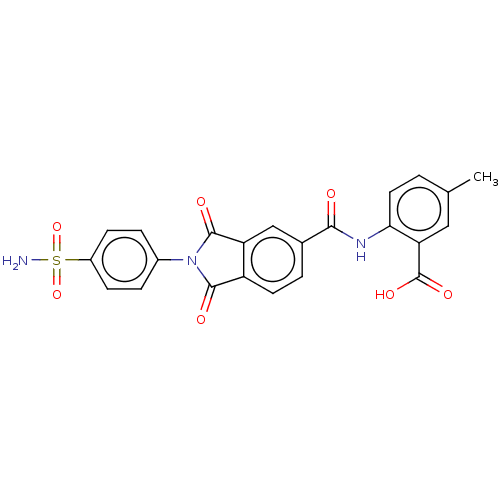 (CHEMBL4063781) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50256774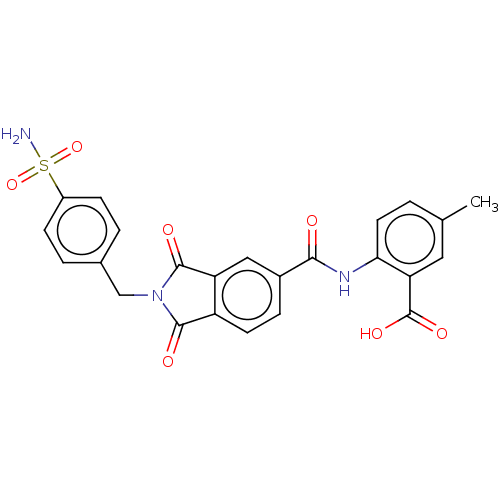 (CHEMBL4075621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50256769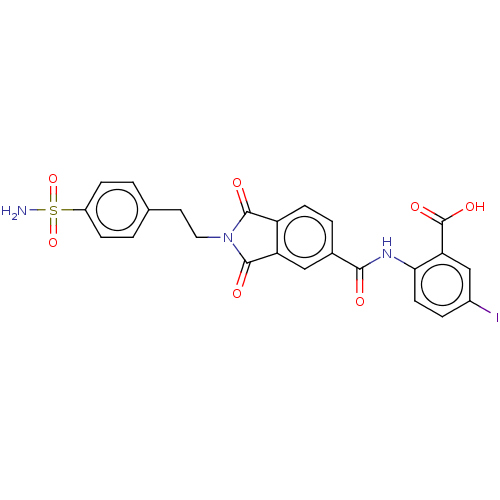 (CHEMBL4094824) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50256788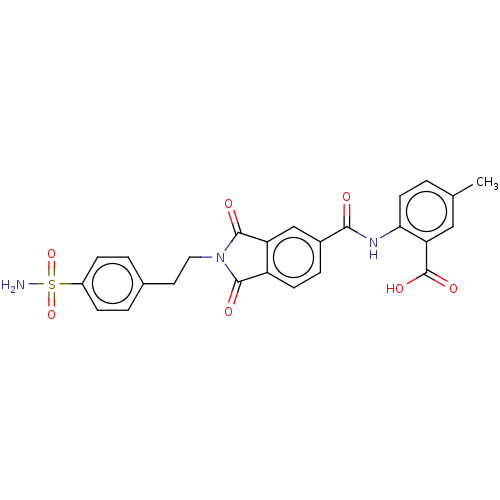 (CHEMBL4068637) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50256789 (CHEMBL4082361) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50256769 (CHEMBL4094824) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50256757 (CHEMBL4063781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50256770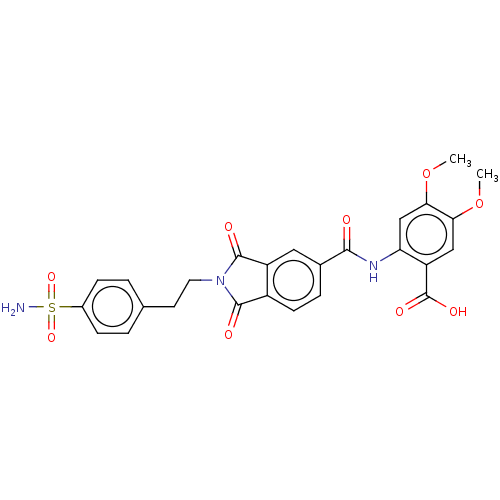 (CHEMBL4067935) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50256775 (CHEMBL4076976) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50256788 (CHEMBL4068637) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50256774 (CHEMBL4075621) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50256758 (CHEMBL4059883) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50256770 (CHEMBL4067935) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50256775 (CHEMBL4076976) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al Kharj, Saudi Arabia; Department of Organic Chemistry, Faculty of Pharmacy, Al-Azhar University, Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 25: 2524-2529 (2017) Article DOI: 10.1016/j.bmc.2017.03.017 BindingDB Entry DOI: 10.7270/Q2TX3HTG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50180630 (CHEMBL3814616) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50180632 (CHEMBL3813998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50180635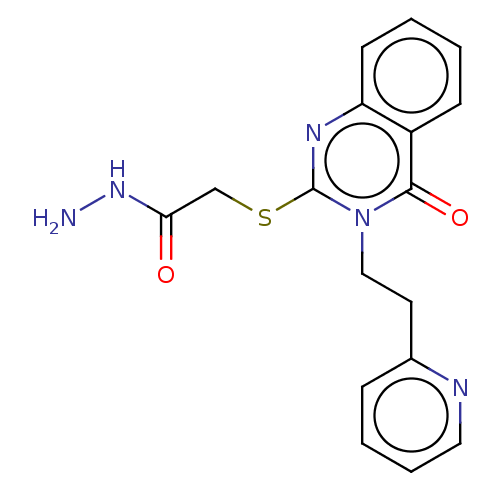 (CHEMBL3815106) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50180633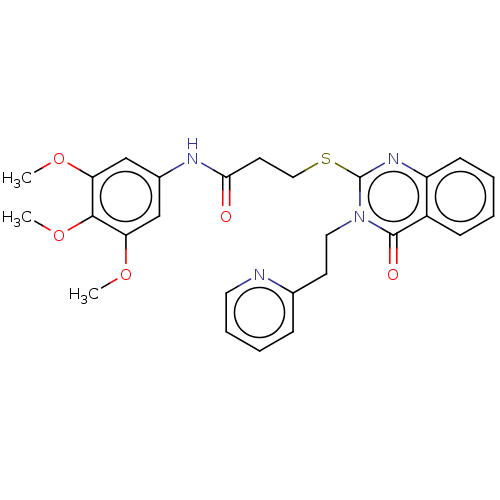 (CHEMBL3814898) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50180631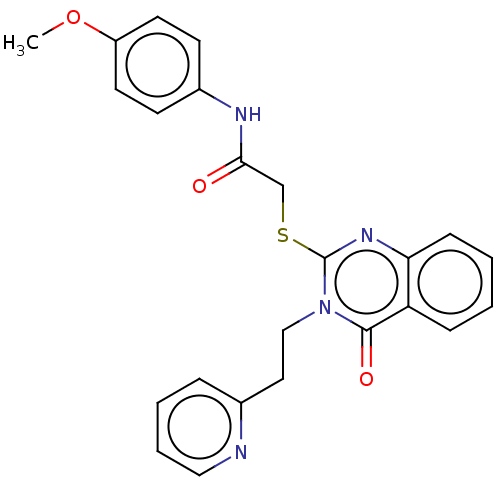 (CHEMBL3813973) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50180629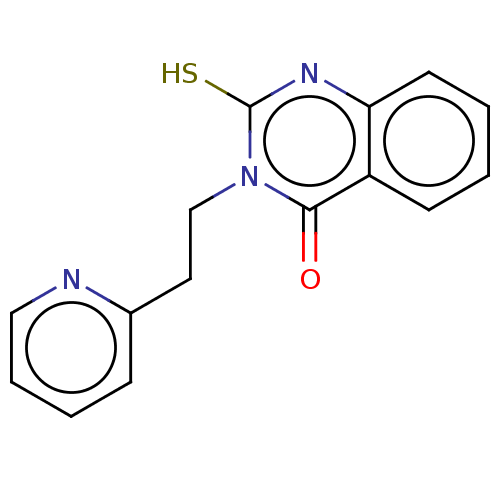 (CHEMBL3814962) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50180634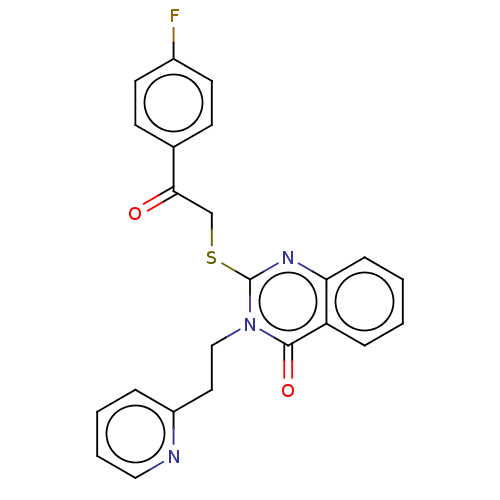 (CHEMBL3814371) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50180635 (CHEMBL3815106) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50180629 (CHEMBL3814962) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50180630 (CHEMBL3814616) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50180633 (CHEMBL3814898) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50180632 (CHEMBL3813998) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50180634 (CHEMBL3814371) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50180631 (CHEMBL3813973) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition by ELISA | Bioorg Med Chem 24: 3818-28 (2016) Article DOI: 10.1016/j.bmc.2016.06.026 BindingDB Entry DOI: 10.7270/Q2QV3PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||