

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50330987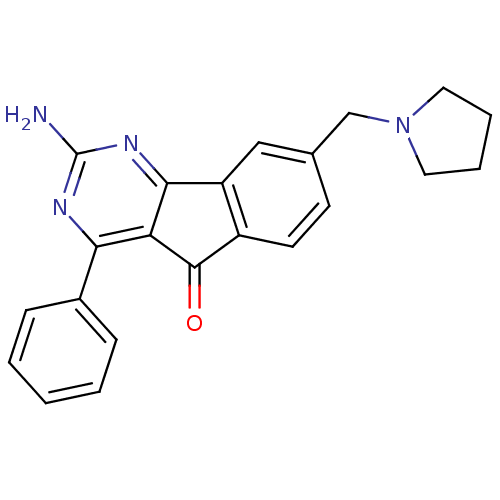 (2-amino-4-phenyl-8-(pyrrolidin-1-ylmethyl)-5H-inde...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A2A receptor | J Med Chem 53: 8104-15 (2010) Checked by Author Article DOI: 10.1021/jm100971t BindingDB Entry DOI: 10.7270/Q2VT1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50330987 (2-amino-4-phenyl-8-(pyrrolidin-1-ylmethyl)-5H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor | J Med Chem 53: 8104-15 (2010) Checked by Author Article DOI: 10.1021/jm100971t BindingDB Entry DOI: 10.7270/Q2VT1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138058 (CHEMBL427478 | {2-[2-(4-Ethyl-3-oxo-3,4-dihydro-2H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138062 ((2-{2-[(S)-4-(6-Fluoro-hexyl)-3-oxo-3,4-dihydro-2H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 570 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138063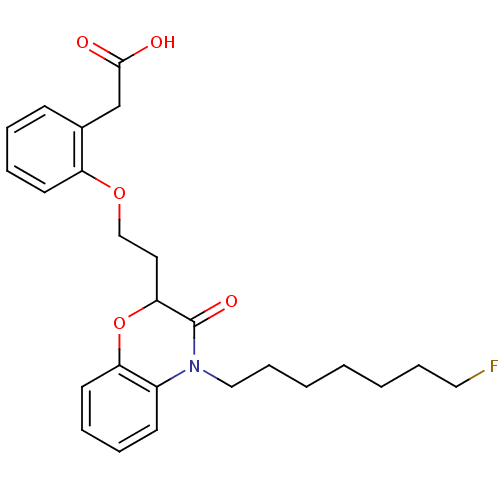 ((2-{2-[4-(7-Fluoro-heptyl)-3-oxo-3,4-dihydro-2H-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 718 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138061 ((2-{2-[4-(3-Cyclopentyl-propyl)-3-oxo-3,4-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138060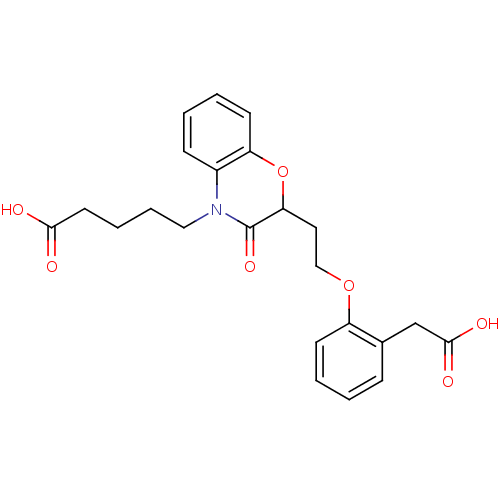 (5-{2-[2-(2-Carboxymethyl-phenoxy)-ethyl]-3-oxo-2,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138065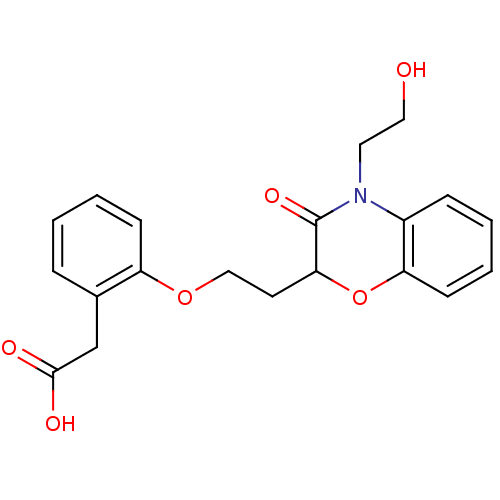 ((2-{2-[4-(2-Hydroxy-ethyl)-3-oxo-3,4-dihydro-2H-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138064 (CHEMBL178536 | {2-[2-(4-Octyl-3-oxo-3,4-dihydro-2H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138066 ((2-{2-[4-(5,5-Difluoro-hexyl)-3-oxo-3,4-dihydro-2H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 179 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138067 ((2-{2-[4-(4-Methoxy-butyl)-3-oxo-3,4-dihydro-2H-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 274 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138068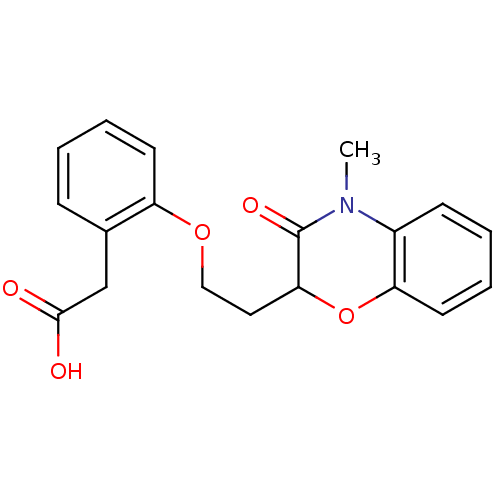 (CHEMBL174167 | {2-[2-(4-Methyl-3-oxo-3,4-dihydro-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138069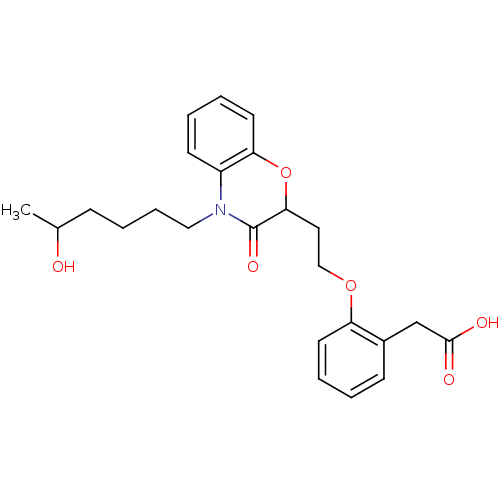 ((2-{2-[4-(5-Hydroxy-hexyl)-3-oxo-3,4-dihydro-2H-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138071 ((2-{2-[(S)-4-(5,5-Difluoro-hexyl)-3-oxo-3,4-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138070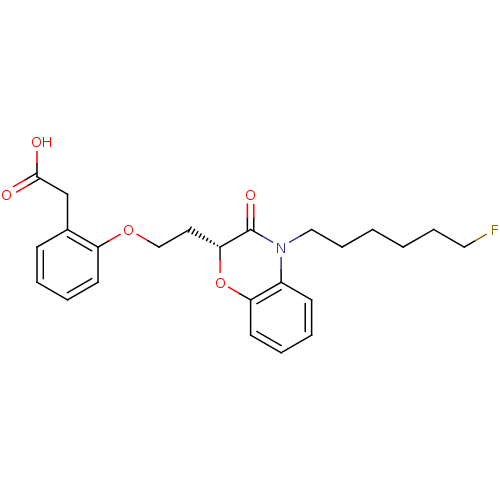 ((2-{2-[(R)-4-(6-Fluoro-hexyl)-3-oxo-3,4-dihydro-2H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 152 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138072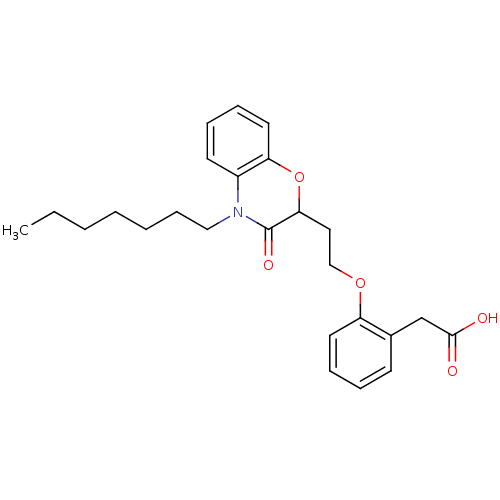 (CHEMBL176770 | {2-[2-(4-Heptyl-3-oxo-3,4-dihydro-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 234 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138073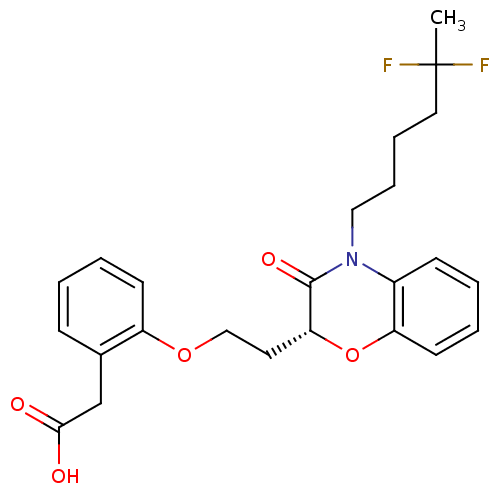 ((2-{2-[(R)-4-(5,5-Difluoro-hexyl)-3-oxo-3,4-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 295 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138076 ((2-{2-[4-(4-Carbamoyl-butyl)-3-oxo-3,4-dihydro-2H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138077 (6-{2-[2-(2-Carboxymethyl-phenoxy)-ethyl]-3-oxo-2,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138074 ((2-{2-[4-(6-Fluoro-hexyl)-3-oxo-3,4-dihydro-2H-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138075 (CHEMBL174644 | {2-[2-(4-Isopropyl-3-oxo-3,4-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138079 (6-{2-[2-(2-Carboxymethyl-phenoxy)-ethyl]-3-oxo-2,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138078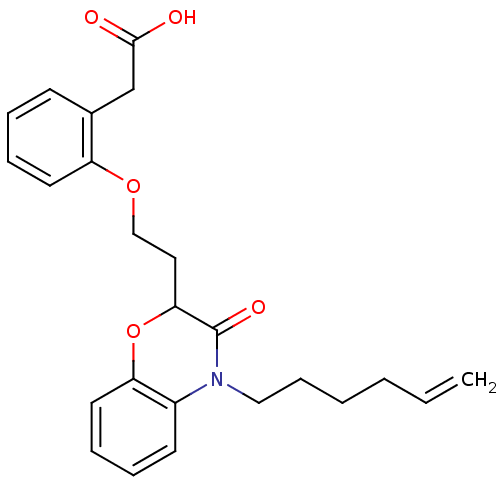 (CHEMBL177830 | {2-[2-(4-Hex-5-enyl-3-oxo-3,4-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 208 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138082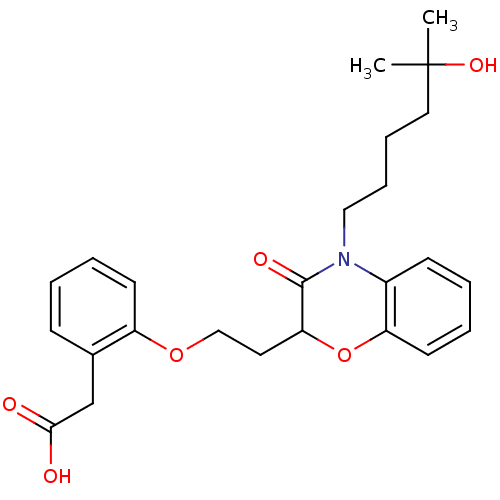 ((2-{2-[4-(5-Hydroxy-5-methyl-hexyl)-3-oxo-3,4-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 644 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138083 ((2-{2-[3-Oxo-4-(5-oxo-hexyl)-3,4-dihydro-2H-benzo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138085 (CHEMBL369054 | {2-[2-(4-Decyl-3-oxo-3,4-dihydro-2H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138080 (CHEMBL176035 | {2-[2-(3-Oxo-4-propylcarbamoylmethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138081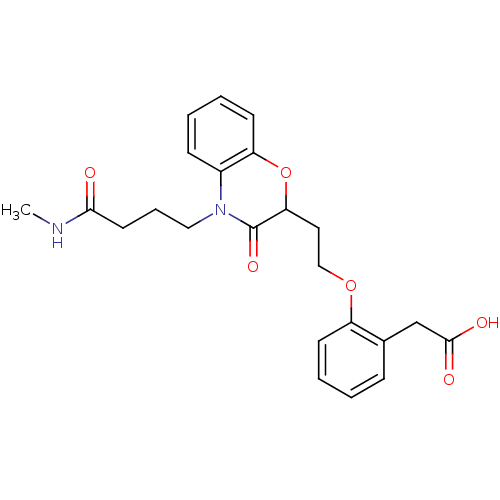 ((2-{2-[4-(3-Methylcarbamoyl-propyl)-3-oxo-3,4-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138084 (CHEMBL177439 | {2-[2-(2-Carboxymethyl-phenoxy)-eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138086 ((2-{2-[4-(5-Cyano-5,5-dimethyl-pentyl)-3-oxo-3,4-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 359 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138087 (CHEMBL174906 | {2-[2-((S)-4-Hexyl-3-oxo-3,4-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138088 ((2-{2-[3-Oxo-4-(6-oxo-heptyl)-3,4-dihydro-2H-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 264 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138090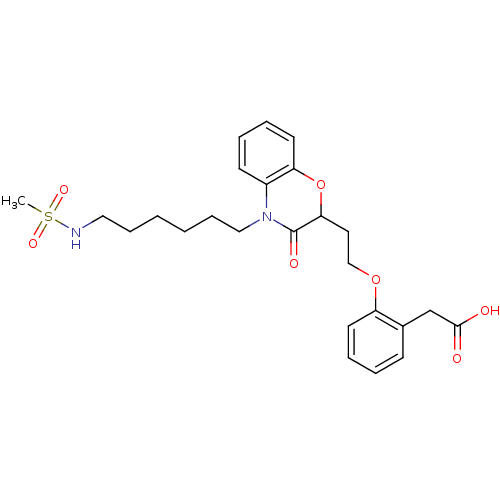 ((2-{2-[4-(6-Methanesulfonylamino-hexyl)-3-oxo-3,4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138089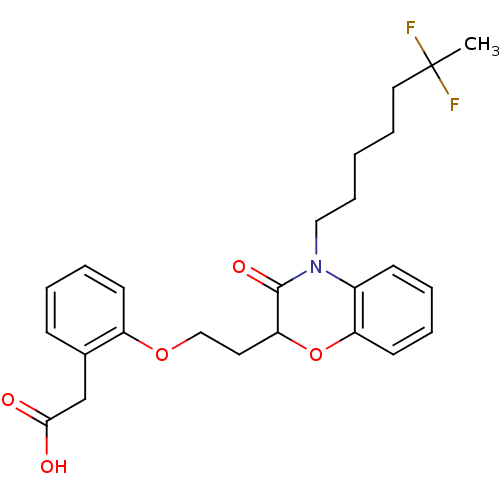 ((2-{2-[4-(6,6-Difluoro-heptyl)-3-oxo-3,4-dihydro-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 534 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138091 ((2-{2-[4-(6-Acetylamino-hexyl)-3-oxo-3,4-dihydro-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138094 (CHEMBL177854 | {2-[2-(3-Oxo-4-pentyl-3,4-dihydro-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 243 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138092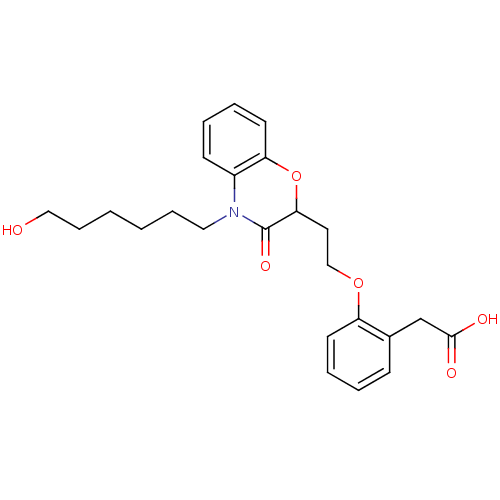 ((2-{2-[4-(6-Hydroxy-hexyl)-3-oxo-3,4-dihydro-2H-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138093 ((2-{2-[4-(5,5-Dimethyl-hexyl)-3-oxo-3,4-dihydro-2H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138096 ((2-{2-[4-(5-Methyl-hexyl)-3-oxo-3,4-dihydro-2H-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138095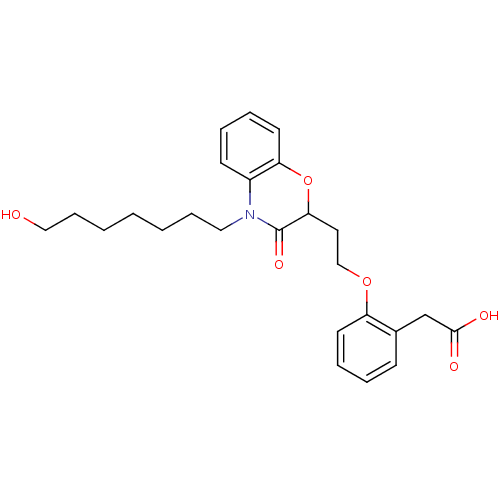 ((2-{2-[4-(7-Hydroxy-heptyl)-3-oxo-3,4-dihydro-2H-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 149 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138100 (CHEMBL177454 | {2-[2-((R)-4-Hexyl-3-oxo-3,4-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138098 ((2-{2-[3-Oxo-4-(2-propylsulfanyl-ethyl)-3,4-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 380 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138097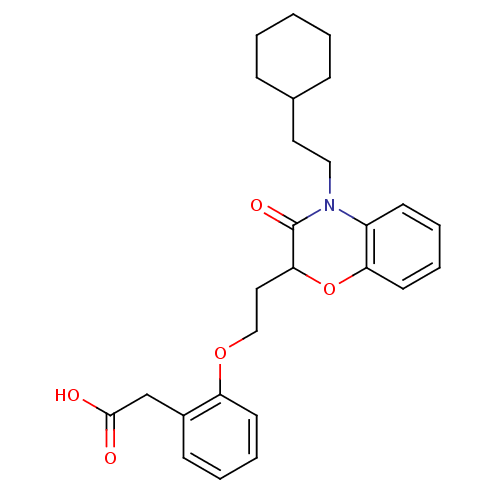 ((2-{2-[4-(2-Cyclohexyl-ethyl)-3-oxo-3,4-dihydro-2H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138099 (CHEMBL177395 | {2-[2-(4-Hexyl-3-oxo-3,4-dihydro-2H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138057 ((2-{2-[4-(5-Hydroxy-pentyl)-3-oxo-3,4-dihydro-2H-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138056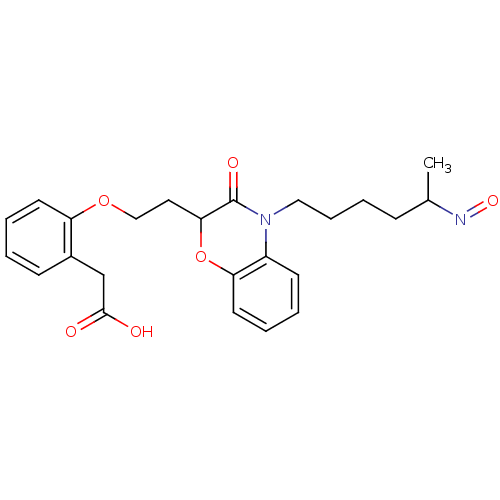 ((2-{2-[4-(5-Hydroxyimino-hexyl)-3-oxo-3,4-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50138059 ((2-{2-[(R)-4-(4-Methoxy-butyl)-3-oxo-3,4-dihydro-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 274 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonistic activity against PPAR (peroxisome proliferator activated receptor gamma) in Suarus chinesis | J Med Chem 47: 196-209 (2003) Article DOI: 10.1021/jm0301888 BindingDB Entry DOI: 10.7270/Q29Z9495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||