 Found 368 hits with Last Name = 'hoefle' and Initial = 'ml'
Found 368 hits with Last Name = 'hoefle' and Initial = 'ml' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027132
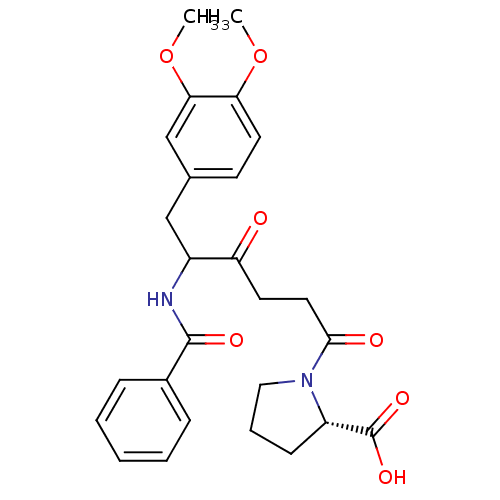
(1-[5-Benzoylamino-6-(3,4-dimethoxy-phenyl)-4-oxo-h...)Show SMILES COc1ccc(CC(NC(=O)c2ccccc2)C(=O)CCC(=O)N2CCC[C@H]2C(O)=O)cc1OC Show InChI InChI=1S/C26H30N2O7/c1-34-22-12-10-17(16-23(22)35-2)15-19(27-25(31)18-7-4-3-5-8-18)21(29)11-13-24(30)28-14-6-9-20(28)26(32)33/h3-5,7-8,10,12,16,19-20H,6,9,11,13-15H2,1-2H3,(H,27,31)(H,32,33)/t19?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027142
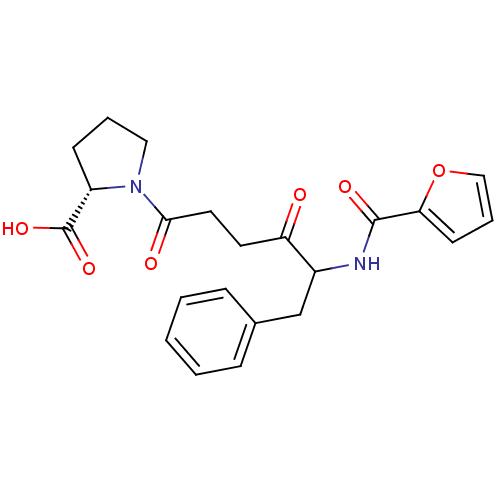
(1-{5-[(Furan-2-carbonyl)-amino]-4-oxo-6-phenyl-hex...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1ccccc1)NC(=O)c1ccco1 Show InChI InChI=1S/C22H24N2O6/c25-18(10-11-20(26)24-12-4-8-17(24)22(28)29)16(14-15-6-2-1-3-7-15)23-21(27)19-9-5-13-30-19/h1-3,5-7,9,13,16-17H,4,8,10-12,14H2,(H,23,27)(H,28,29)/t16?,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50226811

(CHEMBL3349972)Show SMILES [H][C@]12C[C@]([H])(N(C(=O)[C@H](C)N[C@@H](CCc3ccccc3)C(O)=O)[C@@]1([H])CCCC2)C(O)=O Show InChI InChI=1S/C22H30N2O5/c1-14(23-17(21(26)27)12-11-15-7-3-2-4-8-15)20(25)24-18-10-6-5-9-16(18)13-19(24)22(28)29/h2-4,7-8,14,16-19,23H,5-6,9-13H2,1H3,(H,26,27)(H,28,29)/t14-,16-,17-,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50405541
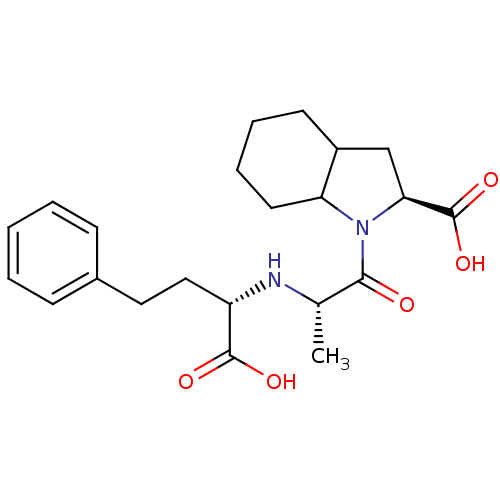
(CHEMBL2079670)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C2CCCCC2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C22H30N2O5/c1-14(23-17(21(26)27)12-11-15-7-3-2-4-8-15)20(25)24-18-10-6-5-9-16(18)13-19(24)22(28)29/h2-4,7-8,14,16-19,23H,5-6,9-13H2,1H3,(H,26,27)(H,28,29)/t14-,16?,17-,18?,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367258
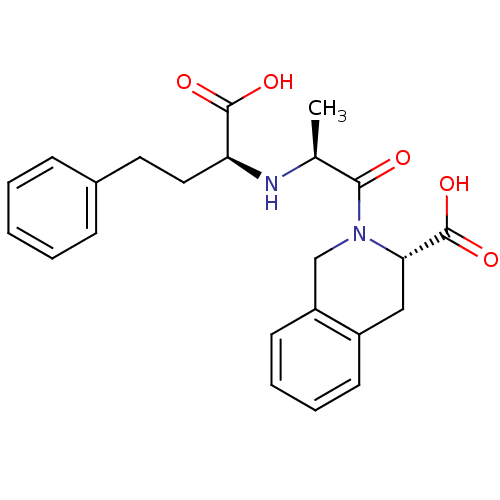
(CI-928 | QUINAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1Cc2ccccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C23H26N2O5/c1-15(24-19(22(27)28)12-11-16-7-3-2-4-8-16)21(26)25-14-18-10-6-5-9-17(18)13-20(25)23(29)30/h2-10,15,19-20,24H,11-14H2,1H3,(H,27,28)(H,29,30)/t15-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021530
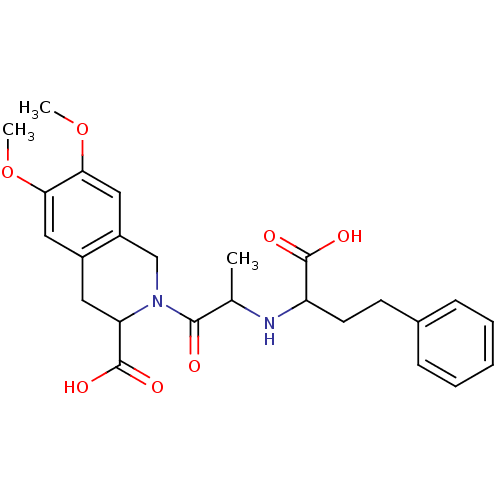
((S,S,S) 2-[2-(1-Carboxy-3-phenyl-propylamino)-prop...)Show SMILES COc1cc2CC(N(Cc2cc1OC)C(=O)C(C)NC(CCc1ccccc1)C(O)=O)C(O)=O Show InChI InChI=1S/C25H30N2O7/c1-15(26-19(24(29)30)10-9-16-7-5-4-6-8-16)23(28)27-14-18-13-22(34-3)21(33-2)12-17(18)11-20(27)25(31)32/h4-8,12-13,15,19-20,26H,9-11,14H2,1-3H3,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021532
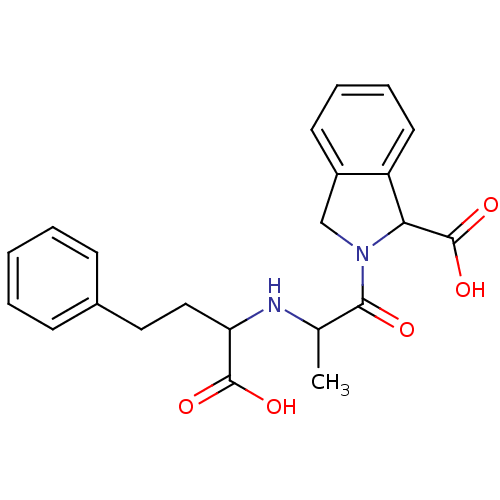
((S,S,S) 2-[2-(1-Carboxy-3-phenyl-propylamino)-prop...)Show SMILES CC(NC(CCc1ccccc1)C(O)=O)C(=O)N1Cc2ccccc2C1C(O)=O Show InChI InChI=1S/C22H24N2O5/c1-14(23-18(21(26)27)12-11-15-7-3-2-4-8-15)20(25)24-13-16-9-5-6-10-17(16)19(24)22(28)29/h2-10,14,18-19,23H,11-13H2,1H3,(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027344
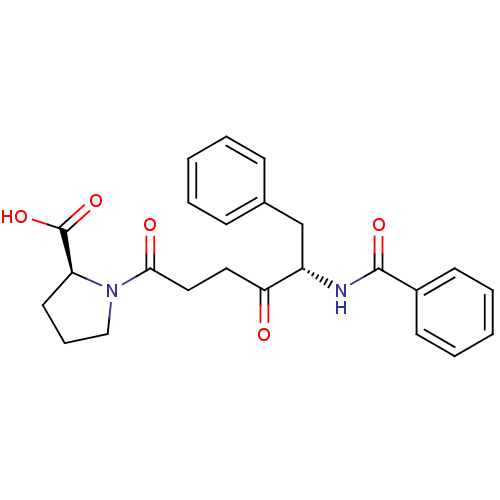
((S)-1-((S)-5-benzamido-4-oxo-6-phenylhexanoyl)pyrr...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O5/c27-21(13-14-22(28)26-15-7-12-20(26)24(30)31)19(16-17-8-3-1-4-9-17)25-23(29)18-10-5-2-6-11-18/h1-6,8-11,19-20H,7,12-16H2,(H,25,29)(H,30,31)/t19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50027141
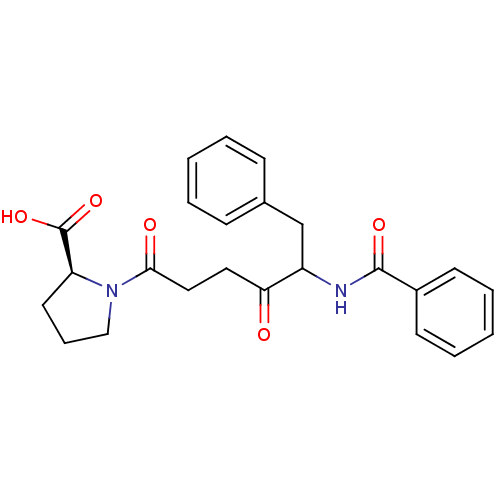
(1-(5-Benzoylamino-4-oxo-6-phenyl-hexanoyl)-pyrroli...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O5/c27-21(13-14-22(28)26-15-7-12-20(26)24(30)31)19(16-17-8-3-1-4-9-17)25-23(29)18-10-5-2-6-11-18/h1-6,8-11,19-20H,7,12-16H2,(H,25,29)(H,30,31)/t19?,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50421824
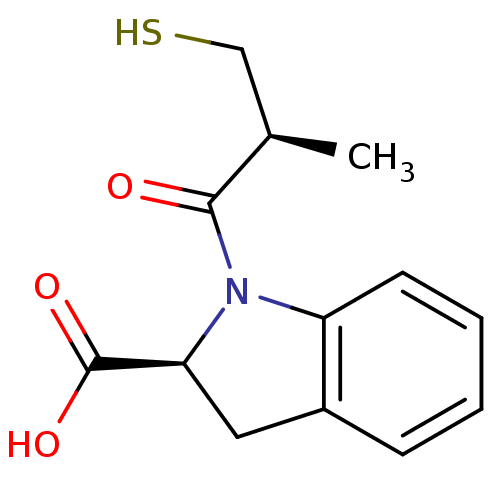
(CHEMBL75752)Show InChI InChI=1S/C13H15NO3S/c1-8(7-18)12(15)14-10-5-3-2-4-9(10)6-11(14)13(16)17/h2-5,8,11,18H,6-7H2,1H3,(H,16,17)/t8-,11+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027147

(1-[5-Benzoylamino-6-(4-hydroxy-phenyl)-4-oxo-hexan...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1ccc(O)cc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O6/c27-18-10-8-16(9-11-18)15-19(25-23(30)17-5-2-1-3-6-17)21(28)12-13-22(29)26-14-4-7-20(26)24(31)32/h1-3,5-6,8-11,19-20,27H,4,7,12-15H2,(H,25,30)(H,31,32)/t19?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50021795
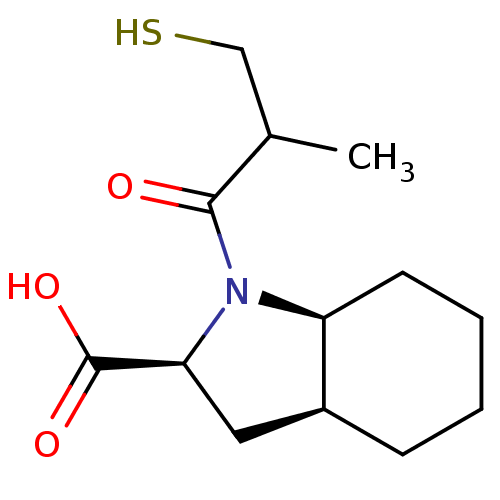
((R,R+S,S) 1-(3-Mercapto-2-methyl-propionyl)-octahy...)Show InChI InChI=1S/C13H21NO3S/c1-8(7-18)12(15)14-10-5-3-2-4-9(10)6-11(14)13(16)17/h8-11,18H,2-7H2,1H3,(H,16,17)/t8?,9-,10-,11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027144
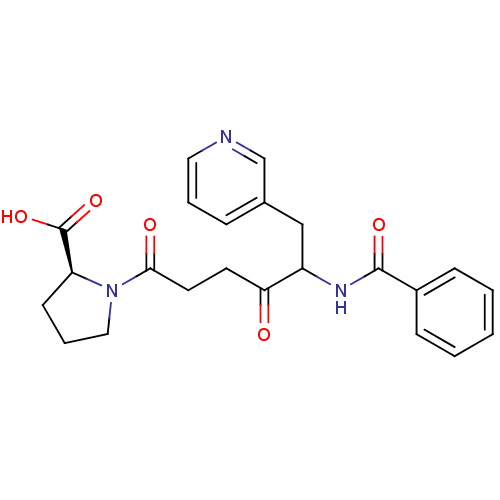
(1-(5-Benzoylamino-4-oxo-6-pyridin-3-yl-hexanoyl)-p...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1cccnc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C23H25N3O5/c27-20(10-11-21(28)26-13-5-9-19(26)23(30)31)18(14-16-6-4-12-24-15-16)25-22(29)17-7-2-1-3-8-17/h1-4,6-8,12,15,18-19H,5,9-11,13-14H2,(H,25,29)(H,30,31)/t18?,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021525

((S,S,S) 2-[2-(1-Carboxy-3-phenyl-propylamino)-prop...)Show SMILES CC(NC(CCc1ccccc1)C(O)=O)C(=O)N1CCc2ccccc2C1C(O)=O Show InChI InChI=1S/C23H26N2O5/c1-15(24-19(22(27)28)12-11-16-7-3-2-4-8-16)21(26)25-14-13-17-9-5-6-10-18(17)20(25)23(29)30/h2-10,15,19-20,24H,11-14H2,1H3,(H,27,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50021808
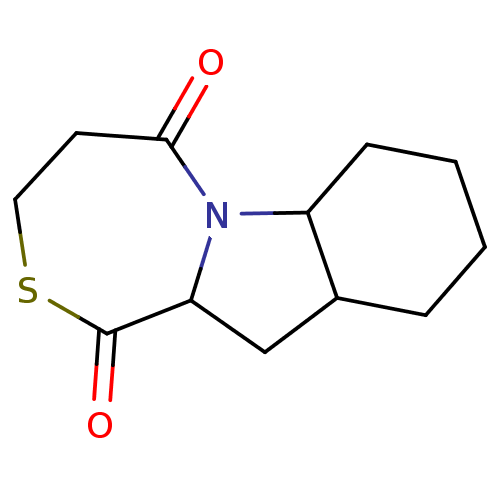
(CHEMBL368598 | Decahydro-8-thia-4b-aza-benzo[a]azu...)Show InChI InChI=1S/C12H17NO2S/c14-11-5-6-16-12(15)10-7-8-3-1-2-4-9(8)13(10)11/h8-10H,1-7H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50005955

(CHEMBL40608 | Octadec-9-enoic acid (2,6-diisopropy...)Show SMILES CCCCCCCC\C=C\CCCCCCCC(=O)Nc1c(cccc1C(C)C)C(C)C Show InChI InChI=1S/C30H51NO/c1-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-24-29(32)31-30-27(25(2)3)22-21-23-28(30)26(4)5/h13-14,21-23,25-26H,6-12,15-20,24H2,1-5H3,(H,31,32)/b14-13+ | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of acyl coenzyme A:cholesterol acyltransferase, in intestinal microsomes isolated from cholesterol-fed rabbits |
J Med Chem 35: 1609-17 (1992)
BindingDB Entry DOI: 10.7270/Q2G44QXN |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011032
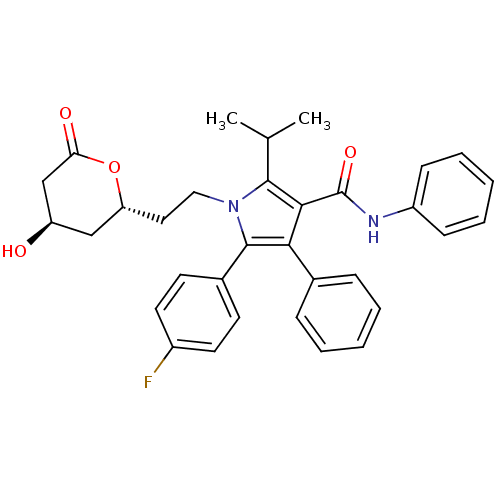
(5-(4-Fluoro-phenyl)-1-[2-(4-hydroxy-6-oxo-tetrahyd...)Show SMILES CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H]1C[C@@H](O)CC(=O)O1)-c1ccccc1 Show InChI InChI=1S/C33H33FN2O4/c1-21(2)31-30(33(39)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-27-19-26(37)20-28(38)40-27/h3-16,21,26-27,37H,17-20H2,1-2H3,(H,35,39)/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase activity in partially purified rat liver |
J Med Chem 34: 357-66 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9H6F |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50368166
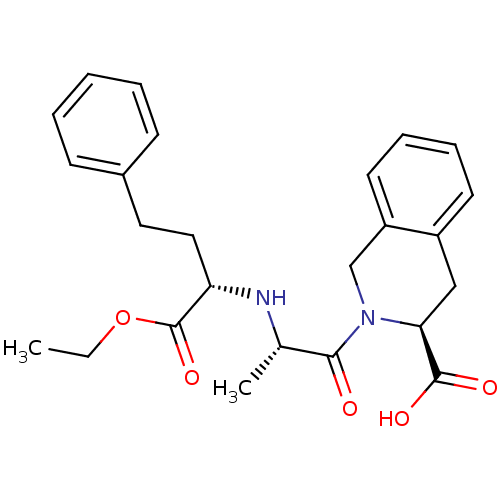
(Accupril | QUINAPRIL)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1Cc2ccccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C25H30N2O5/c1-3-32-25(31)21(14-13-18-9-5-4-6-10-18)26-17(2)23(28)27-16-20-12-8-7-11-19(20)15-22(27)24(29)30/h4-12,17,21-22,26H,3,13-16H2,1-2H3,(H,29,30)/t17-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50021807
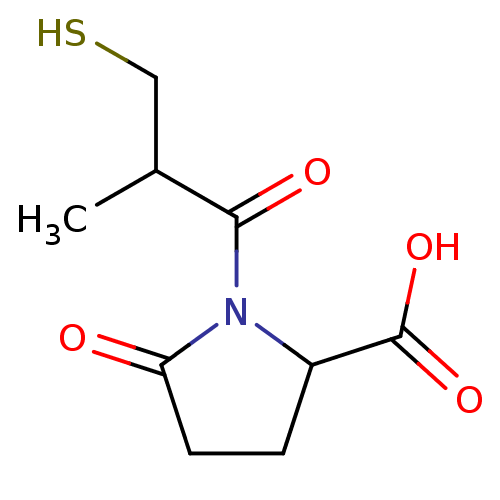
((S,S)1-(3-Mercapto-2-methyl-propionyl)-5-oxo-pyrro...)Show InChI InChI=1S/C9H13NO4S/c1-5(4-15)8(12)10-6(9(13)14)2-3-7(10)11/h5-6,15H,2-4H2,1H3,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027146
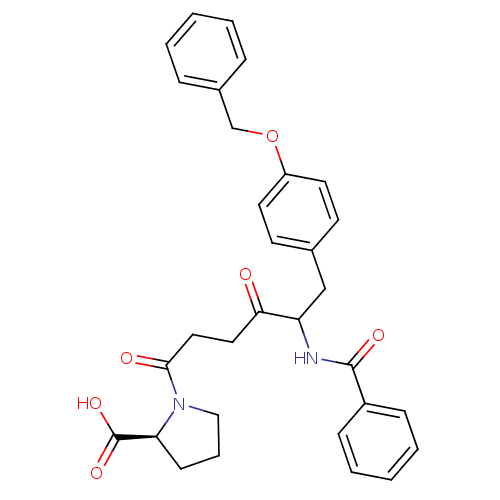
(1-[5-Benzoylamino-6-(4-benzyloxy-phenyl)-4-oxo-hex...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1ccc(OCc2ccccc2)cc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C31H32N2O6/c34-28(17-18-29(35)33-19-7-12-27(33)31(37)38)26(32-30(36)24-10-5-2-6-11-24)20-22-13-15-25(16-14-22)39-21-23-8-3-1-4-9-23/h1-6,8-11,13-16,26-27H,7,12,17-21H2,(H,32,36)(H,37,38)/t26?,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50014669
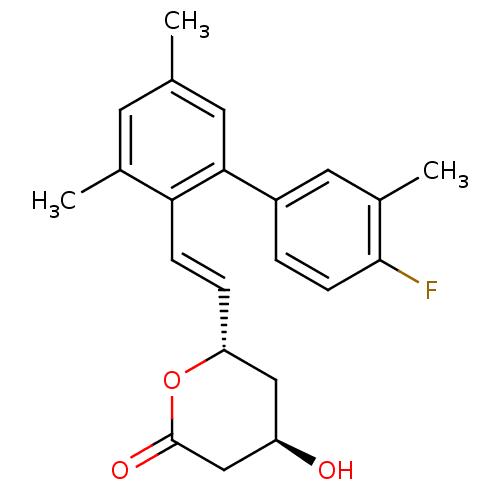
(6-[2-(4'-Fluoro-3,5,3'-trimethyl-biphenyl-2-yl)-vi...)Show SMILES Cc1cc(C)c(\C=C\[C@@H]2C[C@@H](O)CC(=O)O2)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C22H23FO3/c1-13-8-14(2)19(6-5-18-11-17(24)12-22(25)26-18)20(9-13)16-4-7-21(23)15(3)10-16/h4-10,17-18,24H,11-12H2,1-3H3/b6-5+/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit HMG-CoA reductase (HMGR) by cholesterol synthesis inhibition screen (CSI) in rats |
J Med Chem 33: 21-31 (1990)
BindingDB Entry DOI: 10.7270/Q2XG9RR6 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50021801
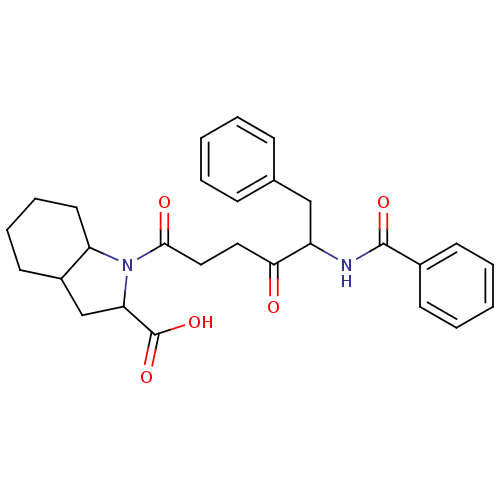
(1-(5-Benzoylamino-4-oxo-6-phenyl-hexanoyl)-octahyd...)Show SMILES OC(=O)C1CC2CCCCC2N1C(=O)CCC(=O)C(Cc1ccccc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C28H32N2O5/c31-25(15-16-26(32)30-23-14-8-7-13-21(23)18-24(30)28(34)35)22(17-19-9-3-1-4-10-19)29-27(33)20-11-5-2-6-12-20/h1-6,9-12,21-24H,7-8,13-18H2,(H,29,33)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50021784
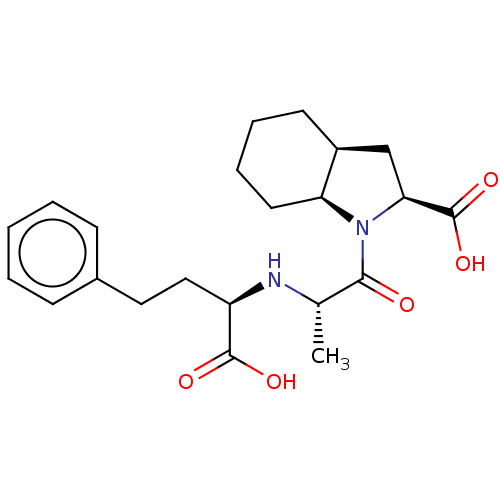
((RSR)1-[2-(1-Carboxy-3-phenyl-propylamino)-propion...)Show SMILES [H][C@]12C[C@]([H])(N(C(=O)[C@H](C)N[C@H](CCc3ccccc3)C(O)=O)[C@@]1([H])CCCC2)C(O)=O Show InChI InChI=1S/C22H30N2O5/c1-14(23-17(21(26)27)12-11-15-7-3-2-4-8-15)20(25)24-18-10-6-5-9-16(18)13-19(24)22(28)29/h2-4,7-8,14,16-19,23H,5-6,9-13H2,1H3,(H,26,27)(H,28,29)/t14?,16-,17?,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50021784

((RSR)1-[2-(1-Carboxy-3-phenyl-propylamino)-propion...)Show SMILES [H][C@]12C[C@]([H])(N(C(=O)[C@H](C)N[C@H](CCc3ccccc3)C(O)=O)[C@@]1([H])CCCC2)C(O)=O Show InChI InChI=1S/C22H30N2O5/c1-14(23-17(21(26)27)12-11-15-7-3-2-4-8-15)20(25)24-18-10-6-5-9-16(18)13-19(24)22(28)29/h2-4,7-8,14,16-19,23H,5-6,9-13H2,1H3,(H,26,27)(H,28,29)/t14?,16-,17?,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of Angiotensin I converting enzyme |
J Med Chem 24: 104-9 (1981)
BindingDB Entry DOI: 10.7270/Q2ZW1MGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50021792
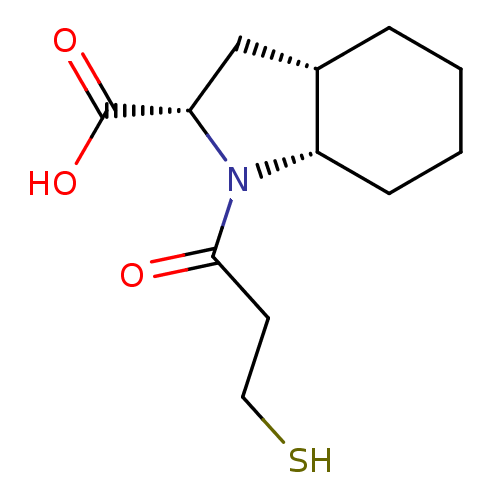
((S)1-(3-Mercapto-propionyl)-octahydro-indole-2-car...)Show InChI InChI=1S/C12H19NO3S/c14-11(5-6-17)13-9-4-2-1-3-8(9)7-10(13)12(15)16/h8-10,17H,1-7H2,(H,15,16)/t8-,9-,10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50367514

(CHEMBL1744311)Show InChI InChI=1S/C12H17NO4S/c14-9(5-6-18)13-10(12(16)17)7-3-1-2-4-8(7)11(13)15/h7-8,10,18H,1-6H2,(H,16,17) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50021792

((S)1-(3-Mercapto-propionyl)-octahydro-indole-2-car...)Show InChI InChI=1S/C12H19NO3S/c14-11(5-6-17)13-9-4-2-1-3-8(9)7-10(13)12(15)16/h8-10,17H,1-7H2,(H,15,16)/t8-,9-,10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50367513

(CHEMBL1744310)Show InChI InChI=1S/C13H19NO4S/c1-7(6-19)11(15)14-10(13(17)18)8-4-2-3-5-9(8)12(14)16/h7-10,19H,2-6H2,1H3,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027154
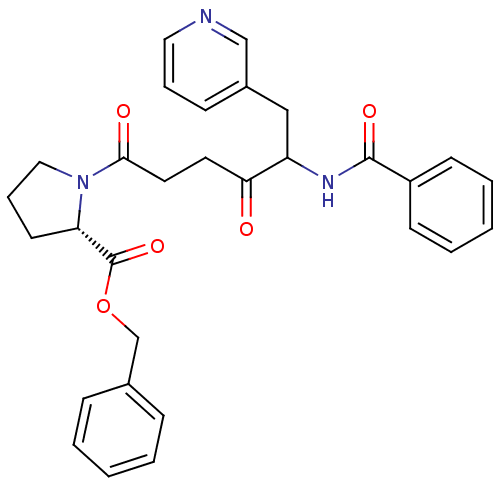
(1-(5-Benzoylamino-4-oxo-6-pyridin-3-yl-hexanoyl)-p...)Show SMILES O=C(CCC(=O)N1CCC[C@H]1C(=O)OCc1ccccc1)C(Cc1cccnc1)NC(=O)c1ccccc1 Show InChI InChI=1S/C30H31N3O5/c34-27(25(19-23-11-7-17-31-20-23)32-29(36)24-12-5-2-6-13-24)15-16-28(35)33-18-8-14-26(33)30(37)38-21-22-9-3-1-4-10-22/h1-7,9-13,17,20,25-26H,8,14-16,18-19,21H2,(H,32,36)/t25?,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50005994
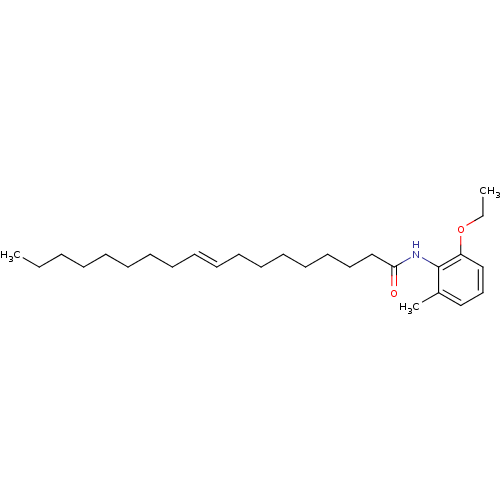
(CHEMBL42998 | Octadec-9-enoic acid (2-ethoxy-6-met...)Show InChI InChI=1S/C27H45NO2/c1-4-6-7-8-9-10-11-12-13-14-15-16-17-18-19-23-26(29)28-27-24(3)21-20-22-25(27)30-5-2/h12-13,20-22H,4-11,14-19,23H2,1-3H3,(H,28,29)/b13-12+ | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of acyl coenzyme A:cholesterol acyltransferase, in intestinal microsomes isolated from cholesterol-fed rabbits |
J Med Chem 35: 1609-17 (1992)
BindingDB Entry DOI: 10.7270/Q2G44QXN |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50005961

(CHEMBL417517 | Octadec-9-enoic acid (2,6-diethyl-p...)Show InChI InChI=1S/C28H47NO/c1-4-7-8-9-10-11-12-13-14-15-16-17-18-19-20-24-27(30)29-28-25(5-2)22-21-23-26(28)6-3/h13-14,21-23H,4-12,15-20,24H2,1-3H3,(H,29,30)/b14-13+ | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of acyl coenzyme A:cholesterol acyltransferase, in intestinal microsomes isolated from cholesterol-fed rabbits |
J Med Chem 35: 1609-17 (1992)
BindingDB Entry DOI: 10.7270/Q2G44QXN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021523
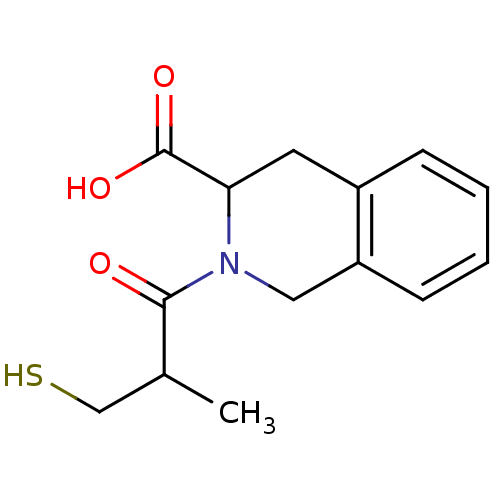
((S,S) 2-(3-Mercapto-2-methyl-propionyl)-1,2,3,4-te...)Show InChI InChI=1S/C14H17NO3S/c1-9(8-19)13(16)15-7-11-5-3-2-4-10(11)6-12(15)14(17)18/h2-5,9,12,19H,6-8H2,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig angiotensin I converting enzyme |
J Med Chem 29: 1953-61 (1986)
BindingDB Entry DOI: 10.7270/Q2PZ59DG |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50405539
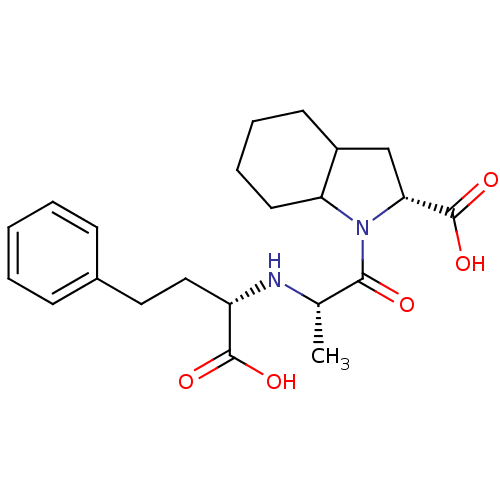
(CHEMBL2052024)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C2CCCCC2C[C@@H]1C(O)=O |r| Show InChI InChI=1S/C22H30N2O5/c1-14(23-17(21(26)27)12-11-15-7-3-2-4-8-15)20(25)24-18-10-6-5-9-16(18)13-19(24)22(28)29/h2-4,7-8,14,16-19,23H,5-6,9-13H2,1H3,(H,26,27)(H,28,29)/t14-,16?,17-,18?,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50027133

(1-{4-Oxo-6-phenyl-5-[(tetrahydro-furan-2-carbonyl)...)Show SMILES OC(=O)[C@@H]1CCCN1C(=O)CCC(=O)C(Cc1ccccc1)NC(=O)C1CCCO1 Show InChI InChI=1S/C22H28N2O6/c25-18(10-11-20(26)24-12-4-8-17(24)22(28)29)16(14-15-6-2-1-3-7-15)23-21(27)19-9-5-13-30-19/h1-3,6-7,16-17,19H,4-5,8-14H2,(H,23,27)(H,28,29)/t16?,17-,19?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% |
J Med Chem 24: 964-9 (1982)
BindingDB Entry DOI: 10.7270/Q2959J4Z |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50005997
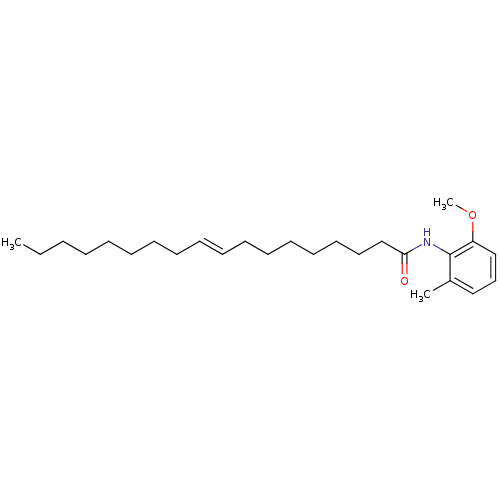
(CHEMBL289033 | Octadec-9-enoic acid (2-methoxy-6-m...)Show InChI InChI=1S/C26H43NO2/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-22-25(28)27-26-23(2)20-19-21-24(26)29-3/h11-12,19-21H,4-10,13-18,22H2,1-3H3,(H,27,28)/b12-11+ | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of acyl coenzyme A:cholesterol acyltransferase, in intestinal microsomes isolated from cholesterol-fed rabbits |
J Med Chem 35: 1609-17 (1992)
BindingDB Entry DOI: 10.7270/Q2G44QXN |
More data for this
Ligand-Target Pair | |
Acyl-CoA:cholesterol acyltransferase
(Oryctolagus cuniculus) | BDBM50005932
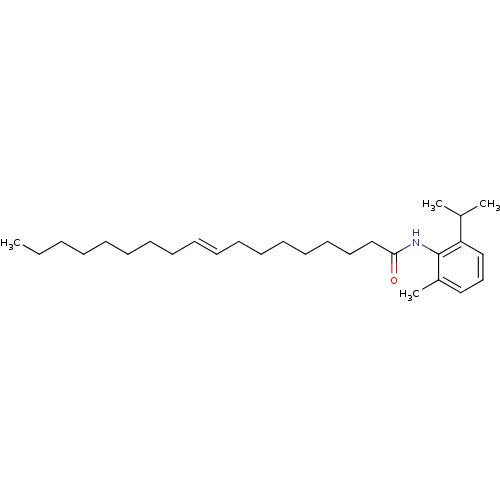
(CHEMBL40527 | Octadec-9-enoic acid (2-isopropyl-6-...)Show InChI InChI=1S/C28H47NO/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-23-27(30)29-28-25(4)21-20-22-26(28)24(2)3/h12-13,20-22,24H,5-11,14-19,23H2,1-4H3,(H,29,30)/b13-12+ | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of acyl coenzyme A:cholesterol acyltransferase, in intestinal microsomes isolated from cholesterol-fed rabbits |
J Med Chem 35: 1609-17 (1992)
BindingDB Entry DOI: 10.7270/Q2G44QXN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50367515

(CHEMBL1744316)Show InChI InChI=1S/C12H19NO3S/c14-10(5-6-17)13-7-8-3-1-2-4-9(8)11(13)12(15)16/h8-9,11,17H,1-7H2,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011032

(5-(4-Fluoro-phenyl)-1-[2-(4-hydroxy-6-oxo-tetrahyd...)Show SMILES CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H]1C[C@@H](O)CC(=O)O1)-c1ccccc1 Show InChI InChI=1S/C33H33FN2O4/c1-21(2)31-30(33(39)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-27-19-26(37)20-28(38)40-27/h3-16,21,26-27,37H,17-20H2,1-2H3,(H,35,39)/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase activity in partially purified rat liver |
J Med Chem 34: 357-66 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9H6F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011032

(5-(4-Fluoro-phenyl)-1-[2-(4-hydroxy-6-oxo-tetrahyd...)Show SMILES CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H]1C[C@@H](O)CC(=O)O1)-c1ccccc1 Show InChI InChI=1S/C33H33FN2O4/c1-21(2)31-30(33(39)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-27-19-26(37)20-28(38)40-27/h3-16,21,26-27,37H,17-20H2,1-2H3,(H,35,39)/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase activity in partially purified rat liver |
J Med Chem 34: 357-66 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9H6F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011036
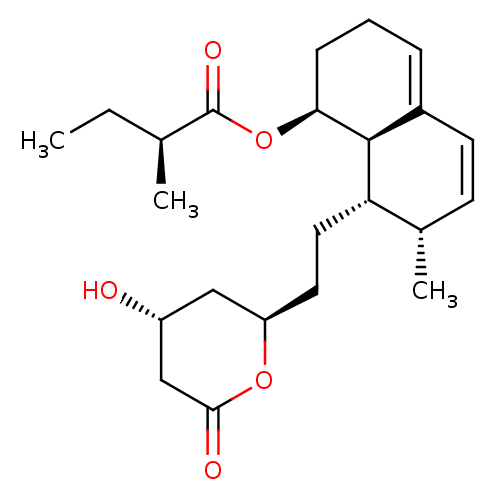
((S)-((1S,7S,8S,8aR)-8-(2-((2R,4R)-4-hydroxy-6-oxo-...)Show SMILES CC[C@H](C)C(=O)O[C@H]1CCC=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:12,t:10| Show InChI InChI=1S/C23H34O5/c1-4-14(2)23(26)28-20-7-5-6-16-9-8-15(3)19(22(16)20)11-10-18-12-17(24)13-21(25)27-18/h6,8-9,14-15,17-20,22,24H,4-5,7,10-13H2,1-3H3/t14-,15-,17+,18+,19-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit HMG-CoA reductase (HMGR) by CoA reductase inhibition screen (COR) in rats |
J Med Chem 33: 21-31 (1990)
BindingDB Entry DOI: 10.7270/Q2XG9RR6 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011036

((S)-((1S,7S,8S,8aR)-8-(2-((2R,4R)-4-hydroxy-6-oxo-...)Show SMILES CC[C@H](C)C(=O)O[C@H]1CCC=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:12,t:10| Show InChI InChI=1S/C23H34O5/c1-4-14(2)23(26)28-20-7-5-6-16-9-8-15(3)19(22(16)20)11-10-18-12-17(24)13-21(25)27-18/h6,8-9,14-15,17-20,22,24H,4-5,7,10-13H2,1-3H3/t14-,15-,17+,18+,19-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit HMG-CoA reductase (HMGR) by CoA reductase inhibition screen (COR) in rats |
J Med Chem 33: 21-31 (1990)
BindingDB Entry DOI: 10.7270/Q2XG9RR6 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011036

((S)-((1S,7S,8S,8aR)-8-(2-((2R,4R)-4-hydroxy-6-oxo-...)Show SMILES CC[C@H](C)C(=O)O[C@H]1CCC=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:12,t:10| Show InChI InChI=1S/C23H34O5/c1-4-14(2)23(26)28-20-7-5-6-16-9-8-15(3)19(22(16)20)11-10-18-12-17(24)13-21(25)27-18/h6,8-9,14-15,17-20,22,24H,4-5,7,10-13H2,1-3H3/t14-,15-,17+,18+,19-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit HMG-CoA reductase (HMGR) by CoA reductase inhibition screen (COR) in rats |
J Med Chem 33: 21-31 (1990)
BindingDB Entry DOI: 10.7270/Q2XG9RR6 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50011036

((S)-((1S,7S,8S,8aR)-8-(2-((2R,4R)-4-hydroxy-6-oxo-...)Show SMILES CC[C@H](C)C(=O)O[C@H]1CCC=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:12,t:10| Show InChI InChI=1S/C23H34O5/c1-4-14(2)23(26)28-20-7-5-6-16-9-8-15(3)19(22(16)20)11-10-18-12-17(24)13-21(25)27-18/h6,8-9,14-15,17-20,22,24H,4-5,7,10-13H2,1-3H3/t14-,15-,17+,18+,19-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit HMG-CoA reductase (HMGR) by cholesterol synthesis inhibition screen (CSI) in rats |
J Med Chem 33: 21-31 (1990)
BindingDB Entry DOI: 10.7270/Q2XG9RR6 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50021804

(2-(3-Mercapto-2-methyl-propionyl)-octahydro-isoind...)Show InChI InChI=1S/C13H21NO3S/c1-8(7-18)12(15)14-6-9-4-2-3-5-10(9)11(14)13(16)17/h8-11,18H,2-7H2,1H3,(H,16,17) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat Angiotensin I converting enzyme (ACE), using Hip-Gly-Gly as synthetic substrate. |
J Med Chem 30: 992-8 (1987)
BindingDB Entry DOI: 10.7270/Q29C6Z02 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data