 Found 226 hits with Last Name = 'dimmito' and Initial = 'mp'
Found 226 hits with Last Name = 'dimmito' and Initial = 'mp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50526893

(CHEMBL4586954)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCc2ccccc2CSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)NNC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H60F2N10O10S2.2C2HF3O2/c55-37-13-5-33(6-14-37)23-43-53(75)65-66-54(76)44(24-34-7-15-38(56)16-8-34)62-48(70)26-60-52(74)46(64-50(72)42(58)22-32-11-19-40(68)20-12-32)30-78-28-36-4-2-1-3-35(36)27-77-29-45(51(73)59-25-47(69)61-43)63-49(71)41(57)21-31-9-17-39(67)18-10-31;2*3-2(4,5)1(6)7/h1-20,41-46,67-68H,21-30,57-58H2,(H,59,73)(H,60,74)(H,61,69)(H,62,70)(H,63,71)(H,64,72)(H,65,75)(H,66,76);2*(H,6,7)/t41-,42-,43-,44-,45+,46+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50535399

(CHEMBL4473857)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1C\C=C\C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,t:28| Show InChI InChI=1S/C48H56N10O10.2C2HF3O2/c49-35(23-31-15-19-33(59)20-16-31)43(63)55-37-13-7-8-14-38(56-44(64)36(50)24-32-17-21-34(60)22-18-32)46(66)52-28-42(62)54-40(26-30-11-5-2-6-12-30)48(68)58-57-47(67)39(25-29-9-3-1-4-10-29)53-41(61)27-51-45(37)65;2*3-2(4,5)1(6)7/h1-12,15-22,35-40,59-60H,13-14,23-28,49-50H2,(H,51,65)(H,52,66)(H,53,61)(H,54,62)(H,55,63)(H,56,64)(H,57,67)(H,58,68);2*(H,6,7)/b8-7+;;/t35-,36-,37+,38+,39-,40-;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human delta opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614218
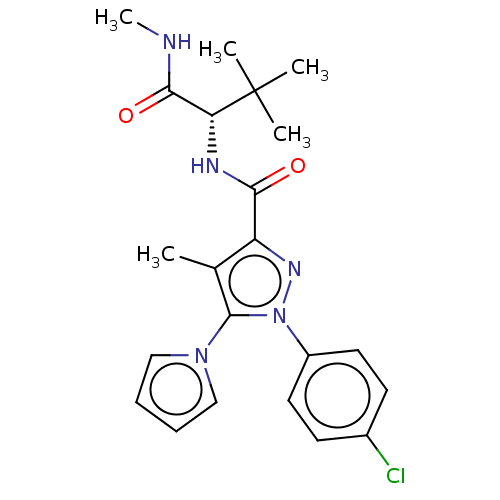
(CHEMBL5275504)Show SMILES CNC(=O)[C@@H](NC(=O)c1nn(c(c1C)-n1cccc1)-c1ccc(Cl)cc1)C(C)(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50535398

(CHEMBL4434948)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1C\C=C/C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,c:28| Show InChI InChI=1S/C48H56N10O10.2C2HF3O2/c49-35(23-31-15-19-33(59)20-16-31)43(63)55-37-13-7-8-14-38(56-44(64)36(50)24-32-17-21-34(60)22-18-32)46(66)52-28-42(62)54-40(26-30-11-5-2-6-12-30)48(68)58-57-47(67)39(25-29-9-3-1-4-10-29)53-41(61)27-51-45(37)65;2*3-2(4,5)1(6)7/h1-12,15-22,35-40,59-60H,13-14,23-28,49-50H2,(H,51,65)(H,52,66)(H,53,61)(H,54,62)(H,55,63)(H,56,64)(H,57,67)(H,58,68);2*(H,6,7)/b8-7-;;/t35-,36-,37+,38+,39-,40-;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human delta opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50526891

(CHEMBL4473632)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCc2ccccc2CSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC1=O |r,wU:27.25,15.13,80.83,44.45,wD:61.62,40.40,(47.76,-44.43,;46.4,-43.65,;46.4,-42.08,;45.04,-44.43,;43.68,-43.65,;45.04,-45.99,;43.68,-45.21,;73.07,-40.39,;71.71,-39.61,;71.71,-38.04,;70.35,-40.39,;68.99,-39.61,;70.35,-41.95,;68.99,-41.17,;48.51,-39.83,;49.84,-39.06,;49.84,-37.52,;48.51,-36.76,;48.52,-35.22,;47.18,-34.45,;45.86,-35.22,;44.52,-34.45,;45.86,-36.76,;47.18,-37.52,;51.18,-39.83,;51.18,-41.37,;52.5,-39.06,;53.83,-39.84,;53.83,-41.4,;52.48,-42.17,;52.47,-43.73,;53.62,-45.44,;52.27,-46.21,;52.26,-47.76,;53.6,-48.55,;54.95,-47.77,;54.96,-46.22,;57.82,-46.78,;59.17,-45.99,;59.16,-44.43,;60.51,-43.65,;61.84,-44.41,;63.17,-43.64,;63.16,-42.1,;64.5,-44.39,;65.82,-43.62,;64.5,-45.93,;65.86,-46.71,;65.86,-48.27,;67.22,-49.04,;68.56,-48.26,;69.92,-49.02,;68.54,-46.69,;67.19,-45.92,;60.5,-42.11,;61.82,-41.33,;59.16,-41.35,;59.15,-39.81,;60.49,-39.04,;61.82,-39.8,;60.48,-37.5,;61.8,-36.73,;63.15,-37.49,;64.46,-36.72,;65.79,-37.49,;67.12,-36.71,;67.11,-35.17,;68.43,-34.39,;65.77,-34.41,;64.45,-35.19,;61.8,-35.19,;63.13,-34.42,;60.47,-34.43,;59.13,-35.2,;57.8,-34.44,;57.8,-32.9,;59.12,-32.13,;60.46,-32.89,;56.46,-32.15,;56.45,-30.6,;55.14,-32.91,;53.8,-32.16,;53.79,-30.61,;55.12,-29.85,;55.12,-28.32,;53.79,-27.55,;53.78,-26.01,;52.45,-28.34,;52.46,-29.87,;55.14,-34.45,;53.81,-35.23,;52.48,-34.46,;53.82,-36.76,;55.16,-37.52,;55.16,-39.06,;56.49,-39.82,)| Show InChI InChI=1S/C58H66F2N10O10S2.2C2HF3O2/c59-41-13-5-37(6-14-41)27-47-57(79)69-21-23-70(24-22-69)58(80)48(28-38-7-15-42(60)16-8-38)66-52(74)30-64-56(78)50(68-54(76)46(62)26-36-11-19-44(72)20-12-36)34-82-32-40-4-2-1-3-39(40)31-81-33-49(55(77)63-29-51(73)65-47)67-53(75)45(61)25-35-9-17-43(71)18-10-35;2*3-2(4,5)1(6)7/h1-20,45-50,71-72H,21-34,61-62H2,(H,63,77)(H,64,78)(H,65,73)(H,66,74)(H,67,75)(H,68,76);2*(H,6,7)/t45-,46-,47-,48-,49+,50+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50526894

(CHEMBL4539430)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSCc2ccccc2CSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)NNC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C58H68F2N10O10S2.2C2HF3O2/c1-31-17-41(71)18-32(2)43(31)23-45(61)53(75)67-49-29-81-27-37-7-5-6-8-38(37)28-82-30-50(68-54(76)46(62)24-44-33(3)19-42(72)20-34(44)4)56(78)64-26-52(74)66-48(22-36-11-15-40(60)16-12-36)58(80)70-69-57(79)47(65-51(73)25-63-55(49)77)21-35-9-13-39(59)14-10-35;2*3-2(4,5)1(6)7/h5-20,45-50,71-72H,21-30,61-62H2,1-4H3,(H,63,77)(H,64,78)(H,65,73)(H,66,74)(H,67,75)(H,68,76)(H,69,79)(H,70,80);2*(H,6,7)/t45-,46-,47-,48-,49+,50+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50526894

(CHEMBL4539430)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSCc2ccccc2CSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)NNC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C58H68F2N10O10S2.2C2HF3O2/c1-31-17-41(71)18-32(2)43(31)23-45(61)53(75)67-49-29-81-27-37-7-5-6-8-38(37)28-82-30-50(68-54(76)46(62)24-44-33(3)19-42(72)20-34(44)4)56(78)64-26-52(74)66-48(22-36-11-15-40(60)16-12-36)58(80)70-69-57(79)47(65-51(73)25-63-55(49)77)21-35-9-13-39(59)14-10-35;2*3-2(4,5)1(6)7/h5-20,45-50,71-72H,21-30,61-62H2,1-4H3,(H,63,77)(H,64,78)(H,65,73)(H,66,74)(H,67,75)(H,68,76)(H,69,79)(H,70,80);2*(H,6,7)/t45-,46-,47-,48-,49+,50+;;/m0../s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50540615
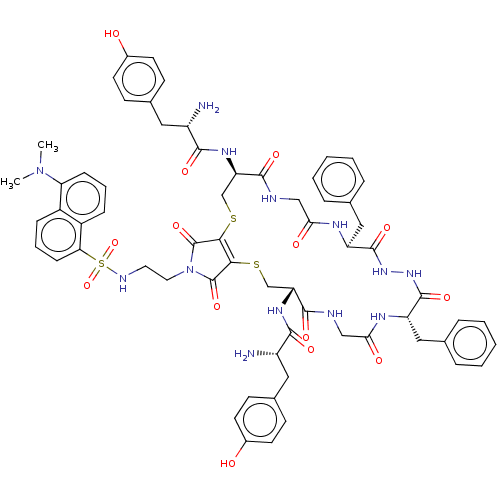
(CHEMBL4638703)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CN(C)c1cccc2c(cccc12)S(=O)(=O)NCCN1C(=O)C2=C(SC[C@@H](NC(=O)[C@@H](N)Cc3ccc(O)cc3)C(=O)NCC(=O)N[C@@H](Cc3ccccc3)C(=O)NNC(=O)[C@H](Cc3ccccc3)NC(=O)CNC(=O)[C@@H](CS2)NC(=O)[C@@H](N)Cc2ccc(O)cc2)C1=O |r,t:36| Show InChI InChI=1S/C64H71N13O14S3.2C2HF3O2/c1-76(2)51-17-9-16-44-43(51)15-10-18-52(44)94(90,91)69-27-28-77-63(88)55-56(64(77)89)93-36-50(73-58(83)46(66)30-40-21-25-42(79)26-22-40)60(85)68-34-54(81)71-48(32-38-13-7-4-8-14-38)62(87)75-74-61(86)47(31-37-11-5-3-6-12-37)70-53(80)33-67-59(84)49(35-92-55)72-57(82)45(65)29-39-19-23-41(78)24-20-39;2*3-2(4,5)1(6)7/h3-26,45-50,69,78-79H,27-36,65-66H2,1-2H3,(H,67,84)(H,68,85)(H,70,80)(H,71,81)(H,72,82)(H,73,83)(H,74,86)(H,75,87);2*(H,6,7)/t45-,46-,47-,48-,49+,50+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine human delta opioid receptor expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method |
ACS Med Chem Lett 11: 720-726 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00569
BindingDB Entry DOI: 10.7270/Q2FR015W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50526893

(CHEMBL4586954)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCc2ccccc2CSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)NNC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H60F2N10O10S2.2C2HF3O2/c55-37-13-5-33(6-14-37)23-43-53(75)65-66-54(76)44(24-34-7-15-38(56)16-8-34)62-48(70)26-60-52(74)46(64-50(72)42(58)22-32-11-19-40(68)20-12-32)30-78-28-36-4-2-1-3-35(36)27-77-29-45(51(73)59-25-47(69)61-43)63-49(71)41(57)21-31-9-17-39(67)18-10-31;2*3-2(4,5)1(6)7/h1-20,41-46,67-68H,21-30,57-58H2,(H,59,73)(H,60,74)(H,61,69)(H,62,70)(H,63,71)(H,64,72)(H,65,75)(H,66,76);2*(H,6,7)/t41-,42-,43-,44-,45+,46+;;/m0../s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614208
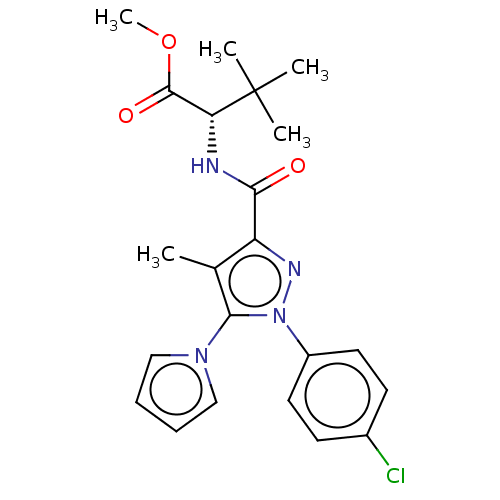
(CHEMBL5291292)Show SMILES COC(=O)[C@@H](NC(=O)c1nn(c(c1C)-n1cccc1)-c1ccc(Cl)cc1)C(C)(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614199
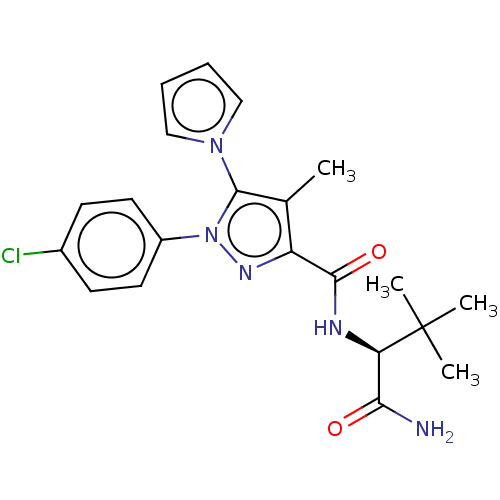
(CHEMBL5271216)Show SMILES Cc1c(nn(c1-n1cccc1)-c1ccc(Cl)cc1)C(=O)N[C@H](C(N)=O)C(C)(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614214
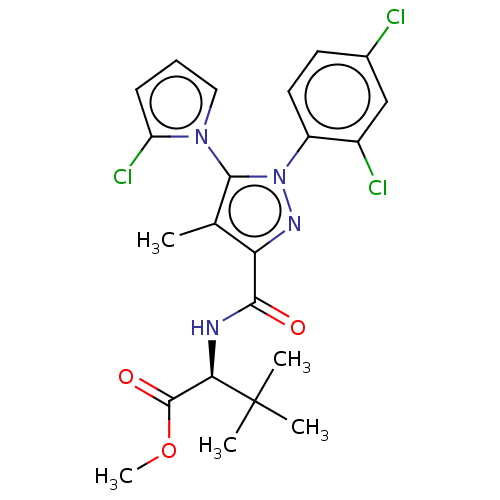
(CHEMBL5288209)Show SMILES COC(=O)[C@@H](NC(=O)c1nn(c(c1C)-n1cccc1Cl)-c1ccc(Cl)cc1Cl)C(C)(C)C |r,wU:4.30,(6.7,4.89,;5.17,5.08,;4.24,3.86,;4.83,2.44,;2.71,4.05,;1.78,2.83,;.25,3.02,;-.35,4.44,;-.68,1.8,;-.24,.32,;-1.51,-.56,;-2.74,.38,;-2.22,1.83,;-3.1,3.09,;-4.21,-.06,;-4.72,-1.51,;-6.26,-1.47,;-6.7,.01,;-5.43,.88,;-5.39,2.42,;-1.55,-2.09,;-2.9,-2.82,;-2.94,-4.36,;-1.63,-5.16,;-1.66,-6.7,;-.27,-4.42,;-.24,-2.88,;1.11,-2.14,;2.11,5.48,;.62,5.08,;1.02,6.57,;3.04,6.7,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50526891

(CHEMBL4473632)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCc2ccccc2CSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC1=O |r,wU:27.25,15.13,80.83,44.45,wD:61.62,40.40,(47.76,-44.43,;46.4,-43.65,;46.4,-42.08,;45.04,-44.43,;43.68,-43.65,;45.04,-45.99,;43.68,-45.21,;73.07,-40.39,;71.71,-39.61,;71.71,-38.04,;70.35,-40.39,;68.99,-39.61,;70.35,-41.95,;68.99,-41.17,;48.51,-39.83,;49.84,-39.06,;49.84,-37.52,;48.51,-36.76,;48.52,-35.22,;47.18,-34.45,;45.86,-35.22,;44.52,-34.45,;45.86,-36.76,;47.18,-37.52,;51.18,-39.83,;51.18,-41.37,;52.5,-39.06,;53.83,-39.84,;53.83,-41.4,;52.48,-42.17,;52.47,-43.73,;53.62,-45.44,;52.27,-46.21,;52.26,-47.76,;53.6,-48.55,;54.95,-47.77,;54.96,-46.22,;57.82,-46.78,;59.17,-45.99,;59.16,-44.43,;60.51,-43.65,;61.84,-44.41,;63.17,-43.64,;63.16,-42.1,;64.5,-44.39,;65.82,-43.62,;64.5,-45.93,;65.86,-46.71,;65.86,-48.27,;67.22,-49.04,;68.56,-48.26,;69.92,-49.02,;68.54,-46.69,;67.19,-45.92,;60.5,-42.11,;61.82,-41.33,;59.16,-41.35,;59.15,-39.81,;60.49,-39.04,;61.82,-39.8,;60.48,-37.5,;61.8,-36.73,;63.15,-37.49,;64.46,-36.72,;65.79,-37.49,;67.12,-36.71,;67.11,-35.17,;68.43,-34.39,;65.77,-34.41,;64.45,-35.19,;61.8,-35.19,;63.13,-34.42,;60.47,-34.43,;59.13,-35.2,;57.8,-34.44,;57.8,-32.9,;59.12,-32.13,;60.46,-32.89,;56.46,-32.15,;56.45,-30.6,;55.14,-32.91,;53.8,-32.16,;53.79,-30.61,;55.12,-29.85,;55.12,-28.32,;53.79,-27.55,;53.78,-26.01,;52.45,-28.34,;52.46,-29.87,;55.14,-34.45,;53.81,-35.23,;52.48,-34.46,;53.82,-36.76,;55.16,-37.52,;55.16,-39.06,;56.49,-39.82,)| Show InChI InChI=1S/C58H66F2N10O10S2.2C2HF3O2/c59-41-13-5-37(6-14-41)27-47-57(79)69-21-23-70(24-22-69)58(80)48(28-38-7-15-42(60)16-8-38)66-52(74)30-64-56(78)50(68-54(76)46(62)26-36-11-19-44(72)20-12-36)34-82-32-40-4-2-1-3-39(40)31-81-33-49(55(77)63-29-51(73)65-47)67-53(75)45(61)25-35-9-17-43(71)18-10-35;2*3-2(4,5)1(6)7/h1-20,45-50,71-72H,21-34,61-62H2,(H,63,77)(H,64,78)(H,65,73)(H,66,74)(H,67,75)(H,68,76);2*(H,6,7)/t45-,46-,47-,48-,49+,50+;;/m0../s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000296

(CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614213
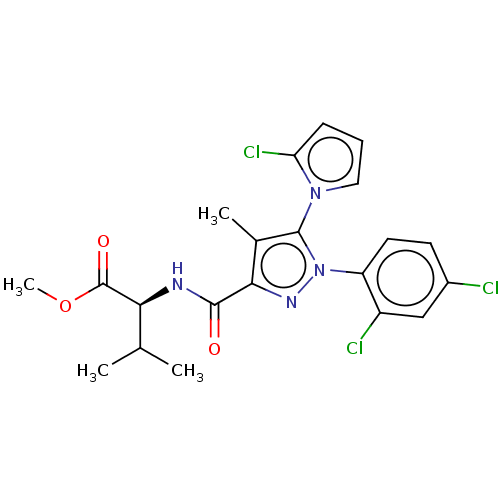
(CHEMBL5281212)Show SMILES COC(=O)[C@@H](NC(=O)c1nn(c(c1C)-n1cccc1Cl)-c1ccc(Cl)cc1Cl)C(C)C |r,wU:4.30,(6.7,4.89,;5.17,5.08,;4.24,3.86,;4.83,2.44,;2.71,4.05,;1.78,2.83,;.25,3.02,;-.35,4.44,;-.68,1.8,;-.24,.32,;-1.51,-.56,;-2.74,.38,;-2.22,1.83,;-3.1,3.09,;-4.21,-.06,;-4.72,-1.51,;-6.26,-1.47,;-6.7,.01,;-5.43,.88,;-5.39,2.42,;-1.55,-2.09,;-2.9,-2.82,;-2.94,-4.36,;-1.63,-5.17,;-1.66,-6.7,;-.27,-4.42,;-.24,-2.88,;1.11,-2.14,;2.11,5.48,;3.04,6.7,;.58,5.67,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000296

(CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human KOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50526892
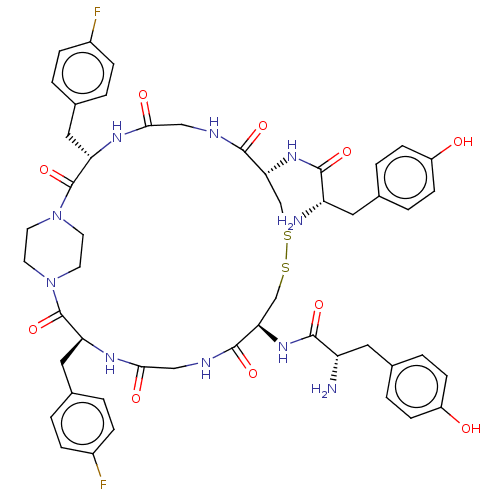
(CHEMBL4551481)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC1=O |r,wU:27.25,15.13,72.74,36.36,wD:53.53,32.31,(34.88,-40.7,;33.52,-39.92,;33.52,-38.35,;32.16,-40.7,;30.8,-39.92,;32.16,-42.27,;30.8,-41.48,;15.86,-46.31,;14.5,-45.53,;14.5,-43.96,;13.14,-46.31,;11.78,-45.53,;13.14,-47.88,;11.77,-47.09,;10.88,-39.84,;12.21,-39.06,;12.21,-37.52,;10.88,-36.76,;10.89,-35.22,;9.55,-34.45,;8.22,-35.22,;6.88,-34.45,;8.23,-36.77,;9.55,-37.52,;13.55,-39.84,;13.55,-41.38,;14.88,-39.06,;16.21,-39.84,;16.2,-41.41,;17.41,-42.61,;19.59,-44.11,;21.55,-44.45,;22.89,-43.66,;24.23,-44.42,;25.56,-43.65,;25.55,-42.11,;26.89,-44.41,;28.22,-43.64,;26.9,-45.95,;28.25,-46.73,;28.25,-48.29,;29.61,-49.06,;30.96,-48.28,;32.32,-49.05,;30.94,-46.71,;29.59,-45.94,;22.89,-42.12,;24.21,-41.34,;21.55,-41.36,;21.54,-39.82,;22.87,-39.05,;24.21,-39.81,;22.87,-37.5,;24.19,-36.73,;25.54,-37.5,;26.86,-36.72,;28.19,-37.49,;29.51,-36.71,;29.51,-35.17,;30.83,-34.39,;28.17,-34.41,;26.84,-35.19,;24.19,-35.19,;25.53,-34.41,;22.85,-34.43,;21.52,-35.2,;20.19,-34.44,;20.18,-32.9,;21.51,-32.13,;22.85,-32.89,;18.84,-32.14,;18.83,-30.59,;17.52,-32.91,;16.18,-32.15,;16.17,-30.61,;17.5,-29.84,;17.5,-28.31,;16.16,-27.54,;16.15,-26,;14.83,-28.33,;14.84,-29.86,;17.52,-34.45,;16.19,-35.23,;14.86,-34.46,;16.2,-36.77,;17.54,-37.52,;17.54,-39.06,;18.88,-39.83,)| Show InChI InChI=1S/C50H58F2N10O10S2.2C2HF3O2/c51-33-9-1-31(2-10-33)23-39-49(71)61-17-19-62(20-18-61)50(72)40(24-32-3-11-34(52)12-4-32)58-44(66)26-56-48(70)42(60-46(68)38(54)22-30-7-15-36(64)16-8-30)28-74-73-27-41(47(69)55-25-43(65)57-39)59-45(67)37(53)21-29-5-13-35(63)14-6-29;2*3-2(4,5)1(6)7/h1-16,37-42,63-64H,17-28,53-54H2,(H,55,69)(H,56,70)(H,57,65)(H,58,66)(H,59,67)(H,60,68);2*(H,6,7)/t37-,38-,39-,40-,41+,42+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614216
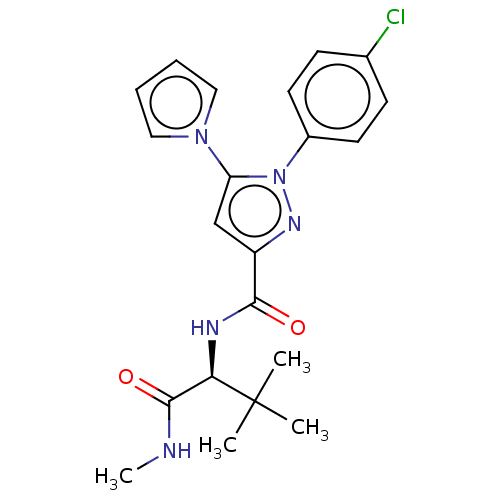
(CHEMBL5280205)Show SMILES CNC(=O)[C@@H](NC(=O)c1cc(-n2cccc2)n(n1)-c1ccc(Cl)cc1)C(C)(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614194
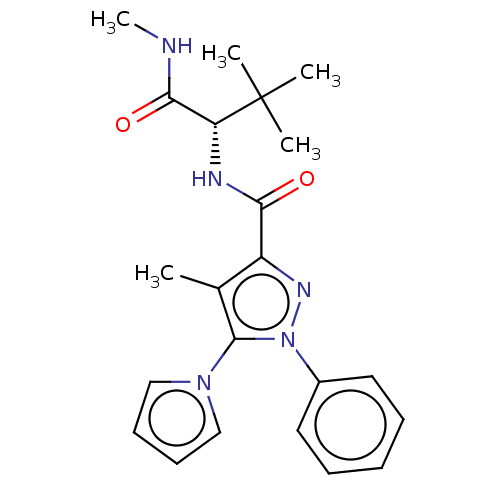
(CHEMBL5289769)Show SMILES CNC(=O)[C@@H](NC(=O)c1nn(c(c1C)-n1cccc1)-c1ccccc1)C(C)(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine human MOR expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method |
ACS Med Chem Lett 11: 720-726 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00569
BindingDB Entry DOI: 10.7270/Q2FR015W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614206
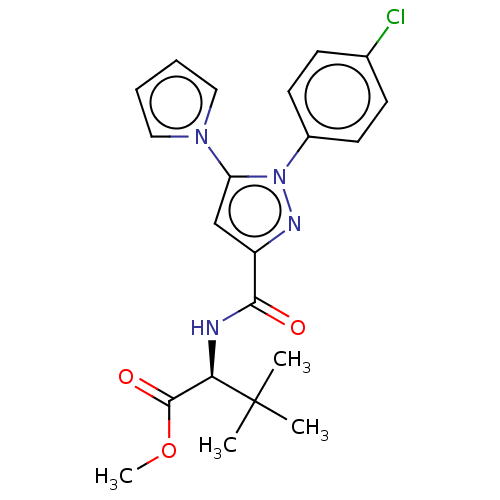
(CHEMBL5268034)Show SMILES COC(=O)[C@@H](NC(=O)c1cc(-n2cccc2)n(n1)-c1ccc(Cl)cc1)C(C)(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614204
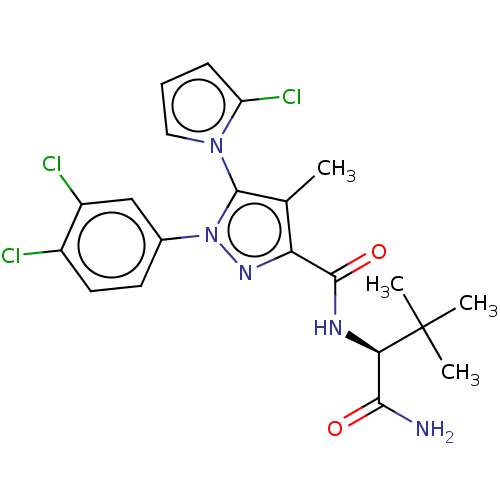
(CHEMBL5280746)Show SMILES Cc1c(nn(c1-n1cccc1Cl)-c1ccc(Cl)c(Cl)c1)C(=O)N[C@H](C(N)=O)C(C)(C)C |r,wU:23.29,(-2.33,3.09,;-1.46,1.83,;.08,1.8,;.52,.32,;-.75,-.56,;-1.97,.38,;-3.44,-.06,;-3.95,-1.51,;-5.49,-1.47,;-5.93,.01,;-4.66,.88,;-4.63,2.42,;-.78,-2.09,;-2.14,-2.82,;-2.18,-4.36,;-.86,-5.17,;-.9,-6.7,;.49,-4.42,;1.81,-5.22,;.53,-2.88,;1.01,3.02,;.42,4.44,;2.54,2.83,;3.47,4.05,;5,3.86,;5.93,5.08,;5.6,2.44,;2.87,5.48,;1.38,5.08,;1.78,6.57,;3.8,6.7,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human mu opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation coun... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50535399

(CHEMBL4473857)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1C\C=C\C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,t:28| Show InChI InChI=1S/C48H56N10O10.2C2HF3O2/c49-35(23-31-15-19-33(59)20-16-31)43(63)55-37-13-7-8-14-38(56-44(64)36(50)24-32-17-21-34(60)22-18-32)46(66)52-28-42(62)54-40(26-30-11-5-2-6-12-30)48(68)58-57-47(67)39(25-29-9-3-1-4-10-29)53-41(61)27-51-45(37)65;2*3-2(4,5)1(6)7/h1-12,15-22,35-40,59-60H,13-14,23-28,49-50H2,(H,51,65)(H,52,66)(H,53,61)(H,54,62)(H,55,63)(H,56,64)(H,57,67)(H,58,68);2*(H,6,7)/b8-7+;;/t35-,36-,37+,38+,39-,40-;;/m0../s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human mu opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation coun... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614207
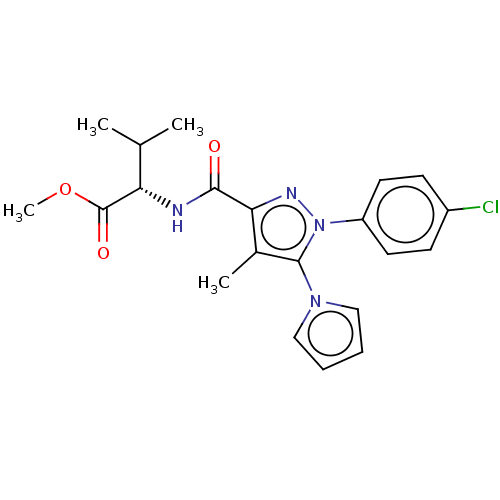
(CHEMBL5288825)Show SMILES COC(=O)[C@@H](NC(=O)c1nn(c(c1C)-n1cccc1)-c1ccc(Cl)cc1)C(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614202
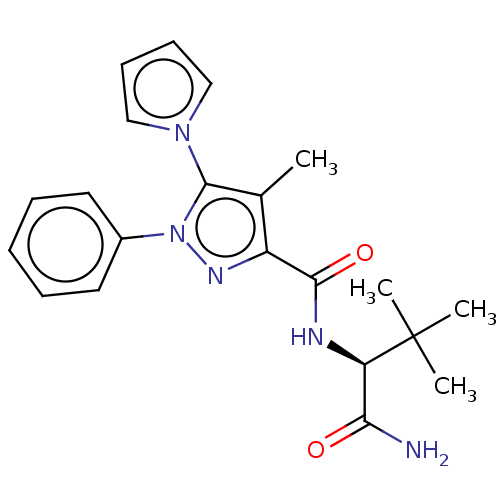
(CHEMBL5268852)Show SMILES Cc1c(nn(c1-n1cccc1)-c1ccccc1)C(=O)N[C@H](C(N)=O)C(C)(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human delta opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50535398

(CHEMBL4434948)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1C\C=C/C[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,c:28| Show InChI InChI=1S/C48H56N10O10.2C2HF3O2/c49-35(23-31-15-19-33(59)20-16-31)43(63)55-37-13-7-8-14-38(56-44(64)36(50)24-32-17-21-34(60)22-18-32)46(66)52-28-42(62)54-40(26-30-11-5-2-6-12-30)48(68)58-57-47(67)39(25-29-9-3-1-4-10-29)53-41(61)27-51-45(37)65;2*3-2(4,5)1(6)7/h1-12,15-22,35-40,59-60H,13-14,23-28,49-50H2,(H,51,65)(H,52,66)(H,53,61)(H,54,62)(H,55,63)(H,56,64)(H,57,67)(H,58,68);2*(H,6,7)/b8-7-;;/t35-,36-,37+,38+,39-,40-;;/m0../s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human mu opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation coun... |
ACS Med Chem Lett 10: 450-456 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00495
BindingDB Entry DOI: 10.7270/Q2N58QWM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50526894

(CHEMBL4539430)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSCc2ccccc2CSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)NNC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C58H68F2N10O10S2.2C2HF3O2/c1-31-17-41(71)18-32(2)43(31)23-45(61)53(75)67-49-29-81-27-37-7-5-6-8-38(37)28-82-30-50(68-54(76)46(62)24-44-33(3)19-42(72)20-34(44)4)56(78)64-26-52(74)66-48(22-36-11-15-40(60)16-12-36)58(80)70-69-57(79)47(65-51(73)25-63-55(49)77)21-35-9-13-39(59)14-10-35;2*3-2(4,5)1(6)7/h5-20,45-50,71-72H,21-30,61-62H2,1-4H3,(H,63,77)(H,64,78)(H,65,73)(H,66,74)(H,67,75)(H,68,76)(H,69,79)(H,70,80);2*(H,6,7)/t45-,46-,47-,48-,49+,50+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human KOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50526892

(CHEMBL4551481)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC1=O |r,wU:27.25,15.13,72.74,36.36,wD:53.53,32.31,(34.88,-40.7,;33.52,-39.92,;33.52,-38.35,;32.16,-40.7,;30.8,-39.92,;32.16,-42.27,;30.8,-41.48,;15.86,-46.31,;14.5,-45.53,;14.5,-43.96,;13.14,-46.31,;11.78,-45.53,;13.14,-47.88,;11.77,-47.09,;10.88,-39.84,;12.21,-39.06,;12.21,-37.52,;10.88,-36.76,;10.89,-35.22,;9.55,-34.45,;8.22,-35.22,;6.88,-34.45,;8.23,-36.77,;9.55,-37.52,;13.55,-39.84,;13.55,-41.38,;14.88,-39.06,;16.21,-39.84,;16.2,-41.41,;17.41,-42.61,;19.59,-44.11,;21.55,-44.45,;22.89,-43.66,;24.23,-44.42,;25.56,-43.65,;25.55,-42.11,;26.89,-44.41,;28.22,-43.64,;26.9,-45.95,;28.25,-46.73,;28.25,-48.29,;29.61,-49.06,;30.96,-48.28,;32.32,-49.05,;30.94,-46.71,;29.59,-45.94,;22.89,-42.12,;24.21,-41.34,;21.55,-41.36,;21.54,-39.82,;22.87,-39.05,;24.21,-39.81,;22.87,-37.5,;24.19,-36.73,;25.54,-37.5,;26.86,-36.72,;28.19,-37.49,;29.51,-36.71,;29.51,-35.17,;30.83,-34.39,;28.17,-34.41,;26.84,-35.19,;24.19,-35.19,;25.53,-34.41,;22.85,-34.43,;21.52,-35.2,;20.19,-34.44,;20.18,-32.9,;21.51,-32.13,;22.85,-32.89,;18.84,-32.14,;18.83,-30.59,;17.52,-32.91,;16.18,-32.15,;16.17,-30.61,;17.5,-29.84,;17.5,-28.31,;16.16,-27.54,;16.15,-26,;14.83,-28.33,;14.84,-29.86,;17.52,-34.45,;16.19,-35.23,;14.86,-34.46,;16.2,-36.77,;17.54,-37.52,;17.54,-39.06,;18.88,-39.83,)| Show InChI InChI=1S/C50H58F2N10O10S2.2C2HF3O2/c51-33-9-1-31(2-10-33)23-39-49(71)61-17-19-62(20-18-61)50(72)40(24-32-3-11-34(52)12-4-32)58-44(66)26-56-48(70)42(60-46(68)38(54)22-30-7-15-36(64)16-8-30)28-74-73-27-41(47(69)55-25-43(65)57-39)59-45(67)37(53)21-29-5-13-35(63)14-6-29;2*3-2(4,5)1(6)7/h1-16,37-42,63-64H,17-28,53-54H2,(H,55,69)(H,56,70)(H,57,65)(H,58,66)(H,59,67)(H,60,68);2*(H,6,7)/t37-,38-,39-,40-,41+,42+;;/m0../s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50540615

(CHEMBL4638703)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CN(C)c1cccc2c(cccc12)S(=O)(=O)NCCN1C(=O)C2=C(SC[C@@H](NC(=O)[C@@H](N)Cc3ccc(O)cc3)C(=O)NCC(=O)N[C@@H](Cc3ccccc3)C(=O)NNC(=O)[C@H](Cc3ccccc3)NC(=O)CNC(=O)[C@@H](CS2)NC(=O)[C@@H](N)Cc2ccc(O)cc2)C1=O |r,t:36| Show InChI InChI=1S/C64H71N13O14S3.2C2HF3O2/c1-76(2)51-17-9-16-44-43(51)15-10-18-52(44)94(90,91)69-27-28-77-63(88)55-56(64(77)89)93-36-50(73-58(83)46(66)30-40-21-25-42(79)26-22-40)60(85)68-34-54(81)71-48(32-38-13-7-4-8-14-38)62(87)75-74-61(86)47(31-37-11-5-3-6-12-37)70-53(80)33-67-59(84)49(35-92-55)72-57(82)45(65)29-39-19-23-41(78)24-20-39;2*3-2(4,5)1(6)7/h3-26,45-50,69,78-79H,27-36,65-66H2,1-2H3,(H,67,84)(H,68,85)(H,70,80)(H,71,81)(H,72,82)(H,73,83)(H,74,86)(H,75,87);2*(H,6,7)/t45-,46-,47-,48-,49+,50+;;/m0../s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine human MOR expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method |
ACS Med Chem Lett 11: 720-726 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00569
BindingDB Entry DOI: 10.7270/Q2FR015W |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50540613
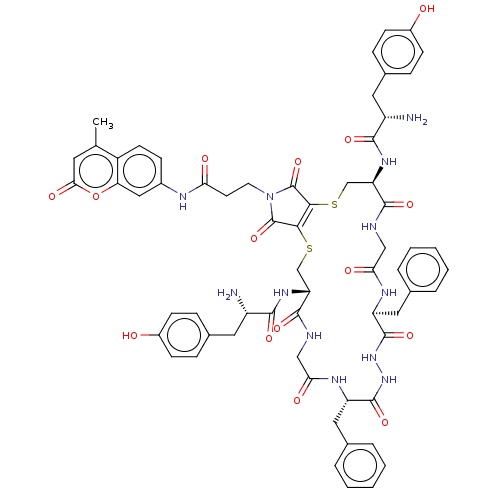
(CHEMBL4641588)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.Cc1cc(=O)oc2cc(NC(=O)CCN3C(=O)C4=C(SC[C@@H](NC(=O)[C@@H](N)Cc5ccc(O)cc5)C(=O)NCC(=O)N[C@@H](Cc5ccccc5)C(=O)NNC(=O)[C@H](Cc5ccccc5)NC(=O)CNC(=O)[C@@H](CS4)NC(=O)[C@@H](N)Cc4ccc(O)cc4)C3=O)ccc12 |r,t:29| Show InChI InChI=1S/C63H66N12O15S2.2C2HF3O2/c1-34-24-53(81)90-49-29-39(16-21-42(34)49)68-50(78)22-23-75-62(88)54-55(63(75)89)92-33-48(72-57(83)44(65)26-38-14-19-41(77)20-15-38)59(85)67-31-52(80)70-46(28-36-10-6-3-7-11-36)61(87)74-73-60(86)45(27-35-8-4-2-5-9-35)69-51(79)30-66-58(84)47(32-91-54)71-56(82)43(64)25-37-12-17-40(76)18-13-37;2*3-2(4,5)1(6)7/h2-21,24,29,43-48,76-77H,22-23,25-28,30-33,64-65H2,1H3,(H,66,84)(H,67,85)(H,68,78)(H,69,79)(H,70,80)(H,71,82)(H,72,83)(H,73,86)(H,74,87);2*(H,6,7)/t43-,44-,45-,46-,47+,48+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine human delta opioid receptor expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method |
ACS Med Chem Lett 11: 720-726 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00569
BindingDB Entry DOI: 10.7270/Q2FR015W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614197
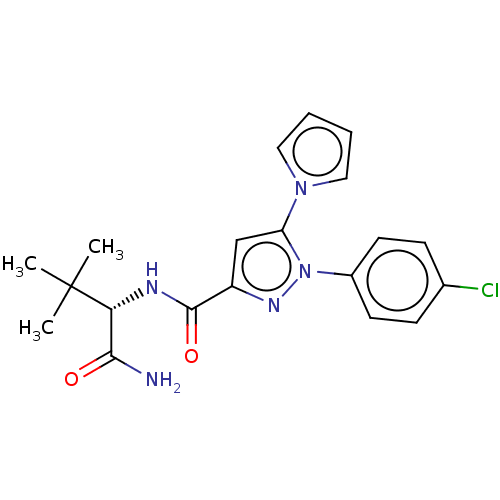
(CHEMBL5284187)Show SMILES CC(C)(C)[C@H](NC(=O)c1cc(-n2cccc2)n(n1)-c1ccc(Cl)cc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine human delta opioid receptor expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method |
ACS Med Chem Lett 11: 720-726 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00569
BindingDB Entry DOI: 10.7270/Q2FR015W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000296

(CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine human kappa opioid receptor expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method |
ACS Med Chem Lett 11: 720-726 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00569
BindingDB Entry DOI: 10.7270/Q2FR015W |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50540614

(CHEMBL4636807)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.Cc1cc(=O)oc2ccc(cc12)N1C(=O)C2=C(SC[C@@H](NC(=O)[C@@H](N)Cc3ccc(O)cc3)C(=O)NCC(=O)N[C@@H](Cc3ccccc3)C(=O)NNC(=O)[C@H](Cc3ccccc3)NC(=O)CNC(=O)[C@@H](CS2)NC(=O)[C@@H](N)Cc2ccc(O)cc2)C1=O |r,t:29| Show InChI InChI=1S/C60H61N11O14S2.2C2HF3O2/c1-32-22-50(76)85-47-21-16-37(27-40(32)47)71-59(83)51-52(60(71)84)87-31-46(68-54(78)42(62)24-36-14-19-39(73)20-15-36)56(80)64-29-49(75)66-44(26-34-10-6-3-7-11-34)58(82)70-69-57(81)43(25-33-8-4-2-5-9-33)65-48(74)28-63-55(79)45(30-86-51)67-53(77)41(61)23-35-12-17-38(72)18-13-35;2*3-2(4,5)1(6)7/h2-22,27,41-46,72-73H,23-26,28-31,61-62H2,1H3,(H,63,79)(H,64,80)(H,65,74)(H,66,75)(H,67,77)(H,68,78)(H,69,81)(H,70,82);2*(H,6,7)/t41-,42-,43-,44-,45+,46+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine human delta opioid receptor expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method |
ACS Med Chem Lett 11: 720-726 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00569
BindingDB Entry DOI: 10.7270/Q2FR015W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50526891

(CHEMBL4473632)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCc2ccccc2CSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC1=O |r,wU:27.25,15.13,80.83,44.45,wD:61.62,40.40,(47.76,-44.43,;46.4,-43.65,;46.4,-42.08,;45.04,-44.43,;43.68,-43.65,;45.04,-45.99,;43.68,-45.21,;73.07,-40.39,;71.71,-39.61,;71.71,-38.04,;70.35,-40.39,;68.99,-39.61,;70.35,-41.95,;68.99,-41.17,;48.51,-39.83,;49.84,-39.06,;49.84,-37.52,;48.51,-36.76,;48.52,-35.22,;47.18,-34.45,;45.86,-35.22,;44.52,-34.45,;45.86,-36.76,;47.18,-37.52,;51.18,-39.83,;51.18,-41.37,;52.5,-39.06,;53.83,-39.84,;53.83,-41.4,;52.48,-42.17,;52.47,-43.73,;53.62,-45.44,;52.27,-46.21,;52.26,-47.76,;53.6,-48.55,;54.95,-47.77,;54.96,-46.22,;57.82,-46.78,;59.17,-45.99,;59.16,-44.43,;60.51,-43.65,;61.84,-44.41,;63.17,-43.64,;63.16,-42.1,;64.5,-44.39,;65.82,-43.62,;64.5,-45.93,;65.86,-46.71,;65.86,-48.27,;67.22,-49.04,;68.56,-48.26,;69.92,-49.02,;68.54,-46.69,;67.19,-45.92,;60.5,-42.11,;61.82,-41.33,;59.16,-41.35,;59.15,-39.81,;60.49,-39.04,;61.82,-39.8,;60.48,-37.5,;61.8,-36.73,;63.15,-37.49,;64.46,-36.72,;65.79,-37.49,;67.12,-36.71,;67.11,-35.17,;68.43,-34.39,;65.77,-34.41,;64.45,-35.19,;61.8,-35.19,;63.13,-34.42,;60.47,-34.43,;59.13,-35.2,;57.8,-34.44,;57.8,-32.9,;59.12,-32.13,;60.46,-32.89,;56.46,-32.15,;56.45,-30.6,;55.14,-32.91,;53.8,-32.16,;53.79,-30.61,;55.12,-29.85,;55.12,-28.32,;53.79,-27.55,;53.78,-26.01,;52.45,-28.34,;52.46,-29.87,;55.14,-34.45,;53.81,-35.23,;52.48,-34.46,;53.82,-36.76,;55.16,-37.52,;55.16,-39.06,;56.49,-39.82,)| Show InChI InChI=1S/C58H66F2N10O10S2.2C2HF3O2/c59-41-13-5-37(6-14-41)27-47-57(79)69-21-23-70(24-22-69)58(80)48(28-38-7-15-42(60)16-8-38)66-52(74)30-64-56(78)50(68-54(76)46(62)26-36-11-19-44(72)20-12-36)34-82-32-40-4-2-1-3-39(40)31-81-33-49(55(77)63-29-51(73)65-47)67-53(75)45(61)25-35-9-17-43(71)18-10-35;2*3-2(4,5)1(6)7/h1-20,45-50,71-72H,21-34,61-62H2,(H,63,77)(H,64,78)(H,65,73)(H,66,74)(H,67,75)(H,68,76);2*(H,6,7)/t45-,46-,47-,48-,49+,50+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human KOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614198
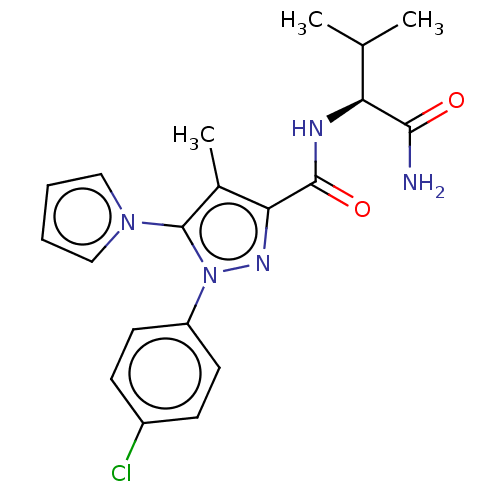
(CHEMBL5285202)Show SMILES CC(C)[C@H](NC(=O)c1nn(c(c1C)-n1cccc1)-c1ccc(Cl)cc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50526892

(CHEMBL4551481)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC1=O |r,wU:27.25,15.13,72.74,36.36,wD:53.53,32.31,(34.88,-40.7,;33.52,-39.92,;33.52,-38.35,;32.16,-40.7,;30.8,-39.92,;32.16,-42.27,;30.8,-41.48,;15.86,-46.31,;14.5,-45.53,;14.5,-43.96,;13.14,-46.31,;11.78,-45.53,;13.14,-47.88,;11.77,-47.09,;10.88,-39.84,;12.21,-39.06,;12.21,-37.52,;10.88,-36.76,;10.89,-35.22,;9.55,-34.45,;8.22,-35.22,;6.88,-34.45,;8.23,-36.77,;9.55,-37.52,;13.55,-39.84,;13.55,-41.38,;14.88,-39.06,;16.21,-39.84,;16.2,-41.41,;17.41,-42.61,;19.59,-44.11,;21.55,-44.45,;22.89,-43.66,;24.23,-44.42,;25.56,-43.65,;25.55,-42.11,;26.89,-44.41,;28.22,-43.64,;26.9,-45.95,;28.25,-46.73,;28.25,-48.29,;29.61,-49.06,;30.96,-48.28,;32.32,-49.05,;30.94,-46.71,;29.59,-45.94,;22.89,-42.12,;24.21,-41.34,;21.55,-41.36,;21.54,-39.82,;22.87,-39.05,;24.21,-39.81,;22.87,-37.5,;24.19,-36.73,;25.54,-37.5,;26.86,-36.72,;28.19,-37.49,;29.51,-36.71,;29.51,-35.17,;30.83,-34.39,;28.17,-34.41,;26.84,-35.19,;24.19,-35.19,;25.53,-34.41,;22.85,-34.43,;21.52,-35.2,;20.19,-34.44,;20.18,-32.9,;21.51,-32.13,;22.85,-32.89,;18.84,-32.14,;18.83,-30.59,;17.52,-32.91,;16.18,-32.15,;16.17,-30.61,;17.5,-29.84,;17.5,-28.31,;16.16,-27.54,;16.15,-26,;14.83,-28.33,;14.84,-29.86,;17.52,-34.45,;16.19,-35.23,;14.86,-34.46,;16.2,-36.77,;17.54,-37.52,;17.54,-39.06,;18.88,-39.83,)| Show InChI InChI=1S/C50H58F2N10O10S2.2C2HF3O2/c51-33-9-1-31(2-10-33)23-39-49(71)61-17-19-62(20-18-61)50(72)40(24-32-3-11-34(52)12-4-32)58-44(66)26-56-48(70)42(60-46(68)38(54)22-30-7-15-36(64)16-8-30)28-74-73-27-41(47(69)55-25-43(65)57-39)59-45(67)37(53)21-29-5-13-35(63)14-6-29;2*3-2(4,5)1(6)7/h1-16,37-42,63-64H,17-28,53-54H2,(H,55,69)(H,56,70)(H,57,65)(H,58,66)(H,59,67)(H,60,68);2*(H,6,7)/t37-,38-,39-,40-,41+,42+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human KOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614217
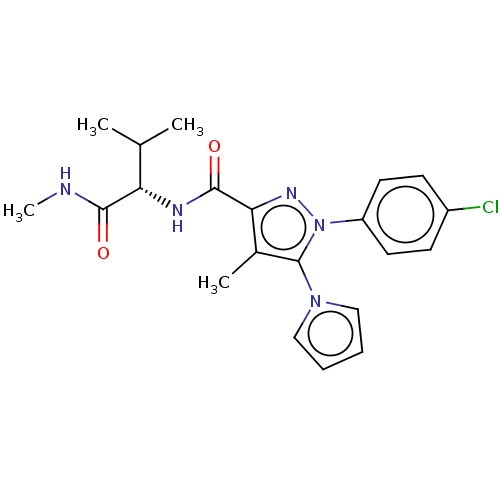
(CHEMBL5272630)Show SMILES CNC(=O)[C@@H](NC(=O)c1nn(c(c1C)-n1cccc1)-c1ccc(Cl)cc1)C(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614195
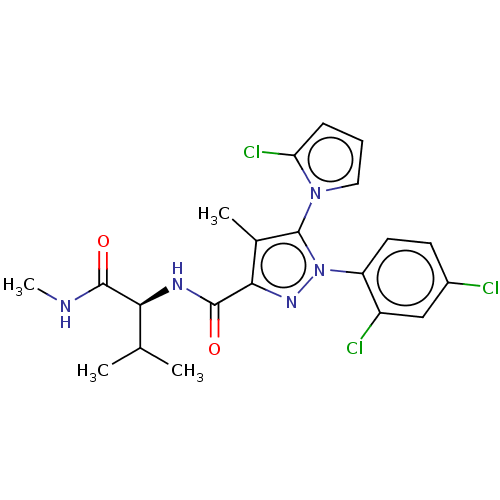
(CHEMBL5276209)Show SMILES CNC(=O)[C@@H](NC(=O)c1nn(c(c1C)-n1cccc1Cl)-c1ccc(Cl)cc1Cl)C(C)C |r,wU:4.30,(6.7,4.89,;5.17,5.08,;4.24,3.86,;4.83,2.44,;2.71,4.05,;1.78,2.83,;.25,3.02,;-.35,4.44,;-.68,1.8,;-.24,.32,;-1.51,-.56,;-2.74,.38,;-2.22,1.83,;-3.1,3.09,;-4.21,-.06,;-4.72,-1.51,;-6.26,-1.47,;-6.7,.01,;-5.43,.88,;-5.39,2.42,;-1.55,-2.09,;-2.9,-2.82,;-2.94,-4.36,;-1.63,-5.17,;-1.66,-6.7,;-.27,-4.42,;-.24,-2.88,;1.11,-2.14,;2.11,5.48,;3.04,6.7,;.58,5.67,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614205
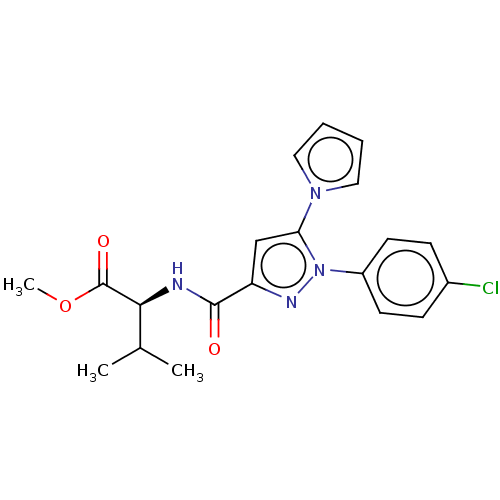
(CHEMBL5281593)Show SMILES COC(=O)[C@@H](NC(=O)c1cc(-n2cccc2)n(n1)-c1ccc(Cl)cc1)C(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 261 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614210
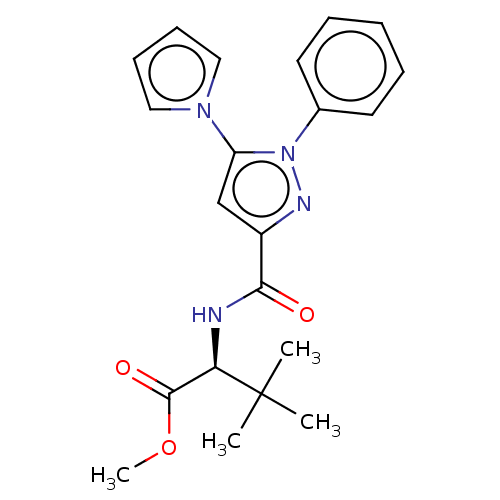
(CHEMBL5279405)Show SMILES COC(=O)[C@@H](NC(=O)c1cc(-n2cccc2)n(n1)-c1ccccc1)C(C)(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 283 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50526893

(CHEMBL4586954)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCc2ccccc2CSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(F)cc2)C(=O)NNC(=O)[C@H](Cc2ccc(F)cc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H60F2N10O10S2.2C2HF3O2/c55-37-13-5-33(6-14-37)23-43-53(75)65-66-54(76)44(24-34-7-15-38(56)16-8-34)62-48(70)26-60-52(74)46(64-50(72)42(58)22-32-11-19-40(68)20-12-32)30-78-28-36-4-2-1-3-35(36)27-77-29-45(51(73)59-25-47(69)61-43)63-49(71)41(57)21-31-9-17-39(67)18-10-31;2*3-2(4,5)1(6)7/h1-20,41-46,67-68H,21-30,57-58H2,(H,59,73)(H,60,74)(H,61,69)(H,62,70)(H,63,71)(H,64,72)(H,65,75)(H,66,76);2*(H,6,7)/t41-,42-,43-,44-,45+,46+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 293 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-diprenorphine from human KOR expressed in CHO cell membranes incubated for 1 hr by scintillation counting method |
J Med Chem 63: 2673-2687 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01963
BindingDB Entry DOI: 10.7270/Q2BG2SFN |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614196
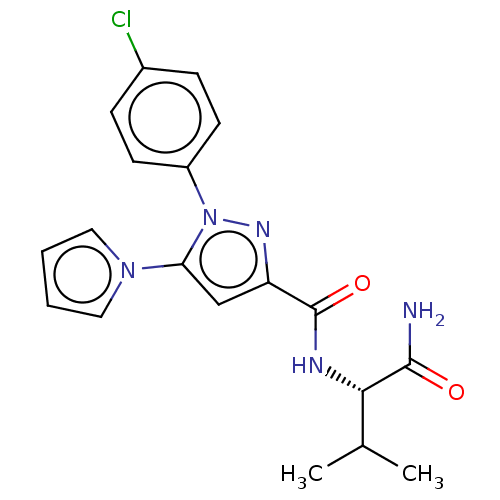
(CHEMBL5275092)Show SMILES CC(C)[C@H](NC(=O)c1cc(-n2cccc2)n(n1)-c1ccc(Cl)cc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50614212
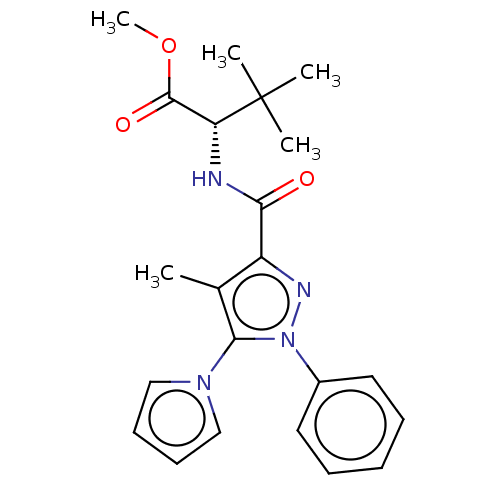
(CHEMBL5275412)Show SMILES COC(=O)[C@@H](NC(=O)c1nn(c(c1C)-n1cccc1)-c1ccccc1)C(C)(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data