

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 min... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50252035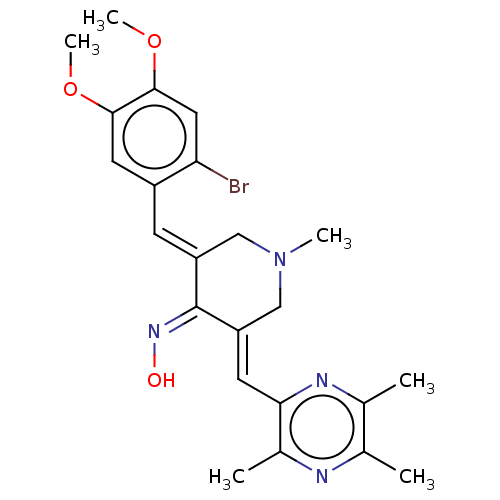 (CHEMBL4064361) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50252033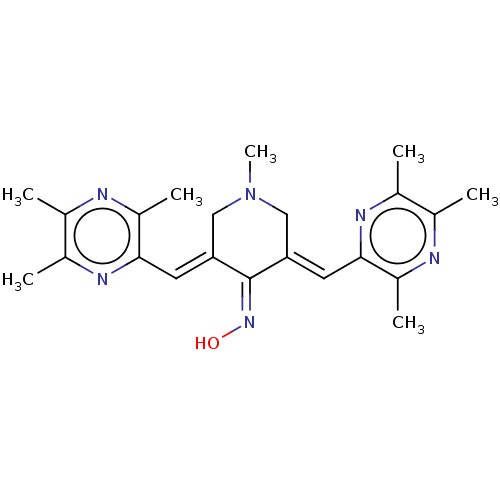 (CHEMBL4078023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161803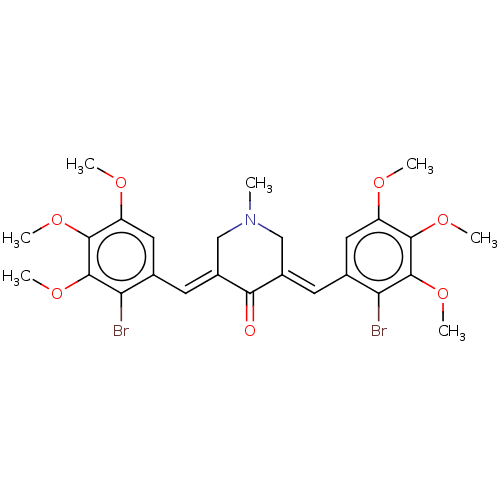 (CHEMBL3785375) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50252089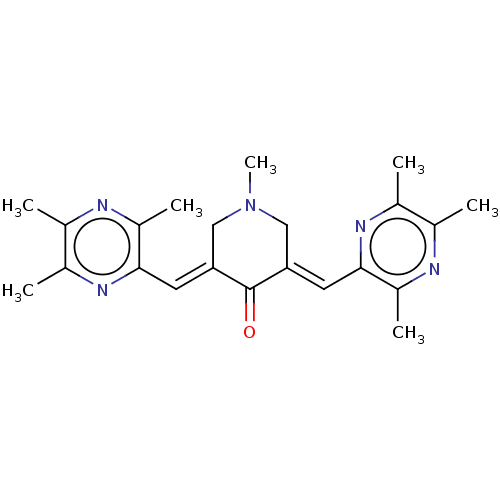 (CHEMBL4083642) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50021461 (CHEMBL3289927) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) after 5 mins by Ellman's method | Eur J Med Chem 83: 355-65 (2014) Article DOI: 10.1016/j.ejmech.2014.06.034 BindingDB Entry DOI: 10.7270/Q2862J10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM203833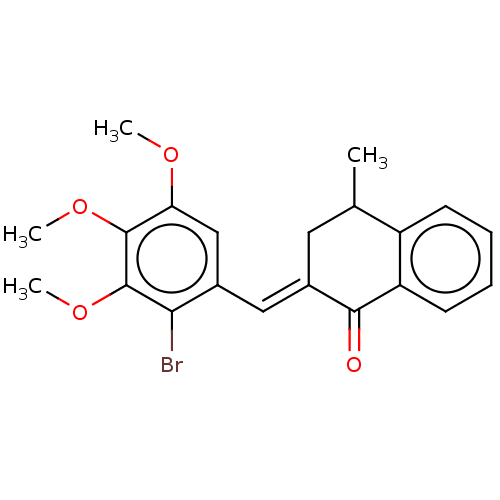 (2-(2-Bromo-3,4,5-trimethoxy-benzylidene)-4-methyl-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50614390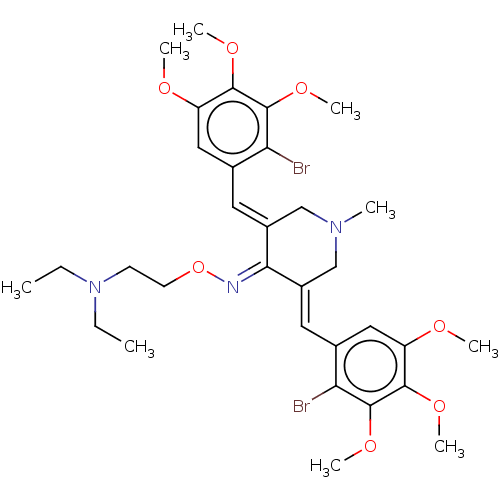 (CHEMBL5271106) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50021471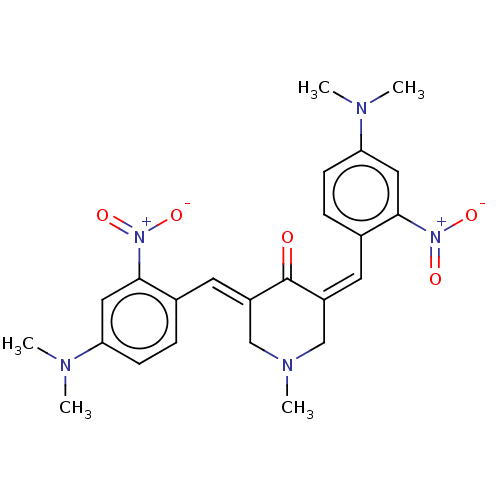 (CHEMBL3289937) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) after 5 mins by Ellman's method | Eur J Med Chem 83: 355-65 (2014) Article DOI: 10.1016/j.ejmech.2014.06.034 BindingDB Entry DOI: 10.7270/Q2862J10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50252090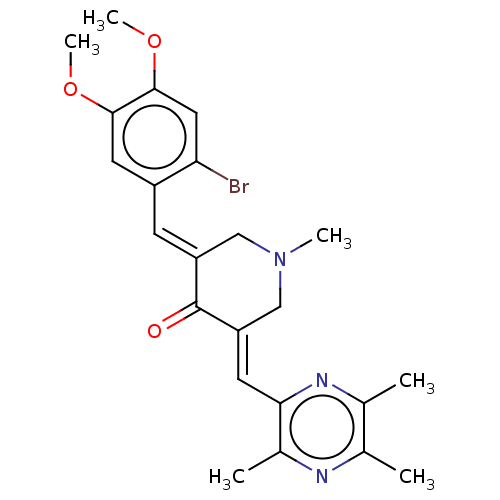 (CHEMBL4063890) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) after 5 mins by Ellman's method | Eur J Med Chem 83: 355-65 (2014) Article DOI: 10.1016/j.ejmech.2014.06.034 BindingDB Entry DOI: 10.7270/Q2862J10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50614382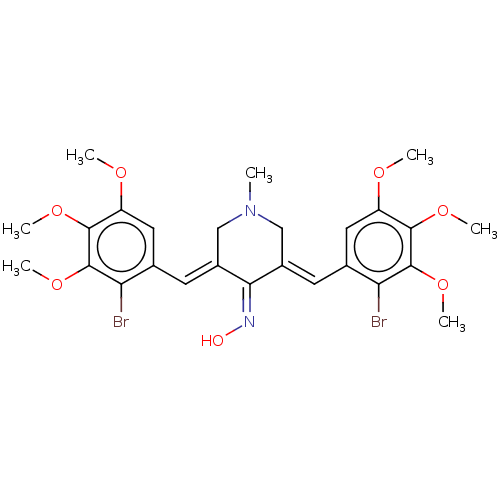 (CHEMBL5279636) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM203832 ((2E)-2-[(2-chloro-3,4-dimethoxyphenyl)methylidene]...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz | Assay Description 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 (ovine) or COX-2(human recombinant), which was preincubated for 10 min in a water bath ... | Chem Biol Drug Des 85: 729-42 (2015) Article DOI: 10.1111/cbdd.12457 BindingDB Entry DOI: 10.7270/Q26W98TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM203851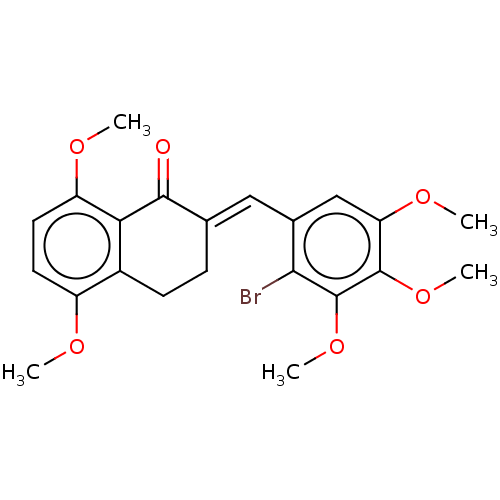 (2-(2-Bromo-3,4,5-trimethoxy-benzylidene)-5,8-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50021461 (CHEMBL3289927) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50021468 (CHEMBL3289934) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM153295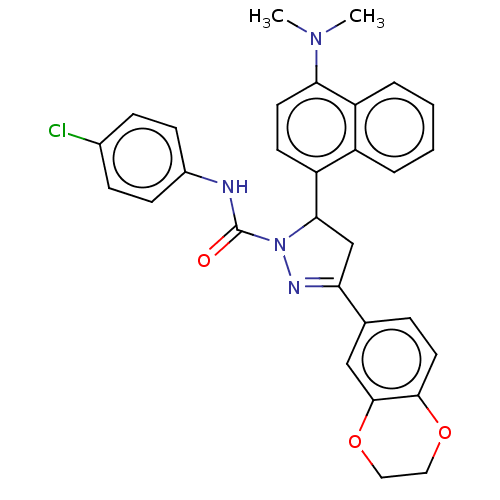 (3-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-5-(4-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz | Assay Description 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 (ovine) or COX-2(human recombinant), which was preincubated for 10 min in a water bath ... | Chem Biol Drug Des 85: 729-42 (2015) Article DOI: 10.1111/cbdd.12457 BindingDB Entry DOI: 10.7270/Q26W98TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50021447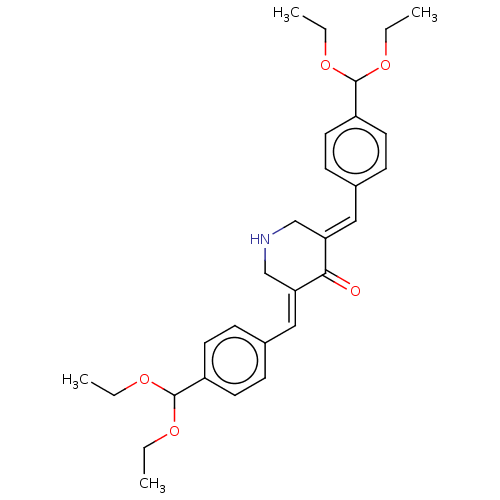 (CHEMBL3289926) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50614381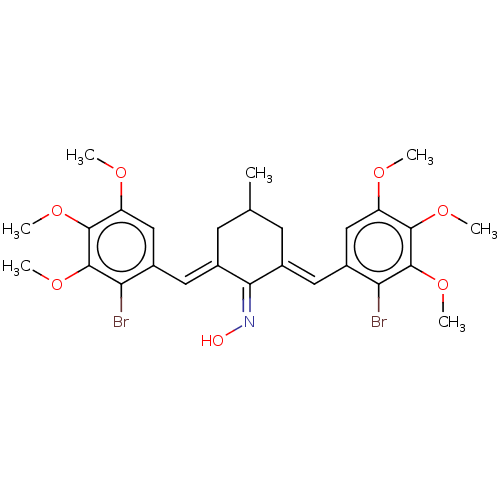 (CHEMBL5275872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161797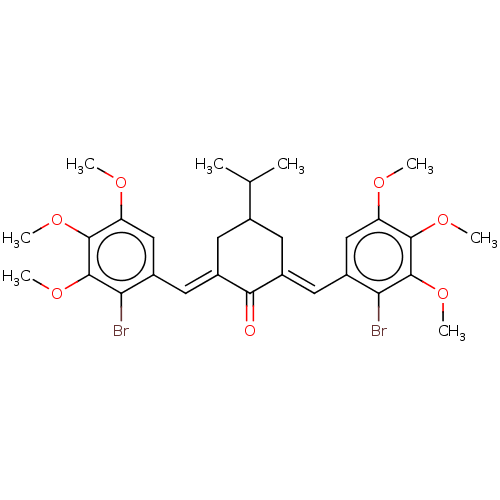 (CHEMBL3785402) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM203860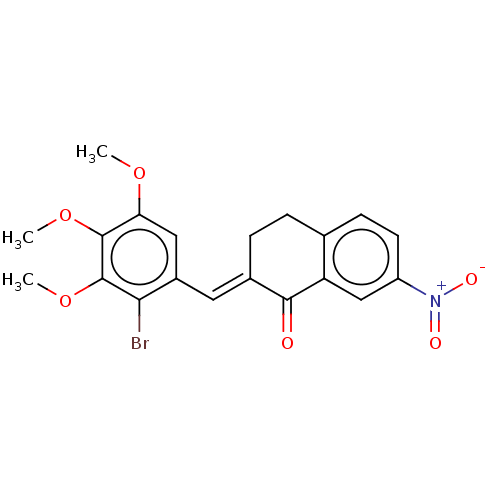 (2-(2-Bromo-3,4,5-trimethoxy-benzylidene)-7-nitro-t...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Wuhan University of Technology | Assay Description This spectrophotometric assay is based on the reaction of 5,5-dithio-bis(2-nitrobenzoic)acid (DTNB) with thiocholine to yield a colored product. Sh... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz | Assay Description Briefly, sn-2ester bond of the substrate 1,2-bis(heptanoylthio)-glycerophosphocholine was hydrolyzed by PLA2-V followed by the exposure of free thiol... | Chem Biol Drug Des 85: 729-42 (2015) Article DOI: 10.1111/cbdd.12457 BindingDB Entry DOI: 10.7270/Q26W98TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161802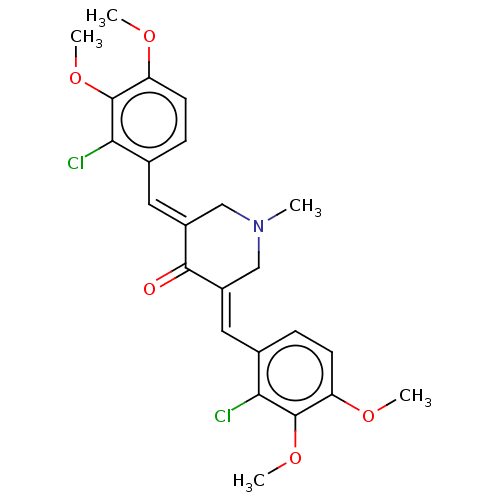 (CHEMBL3787299) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50045848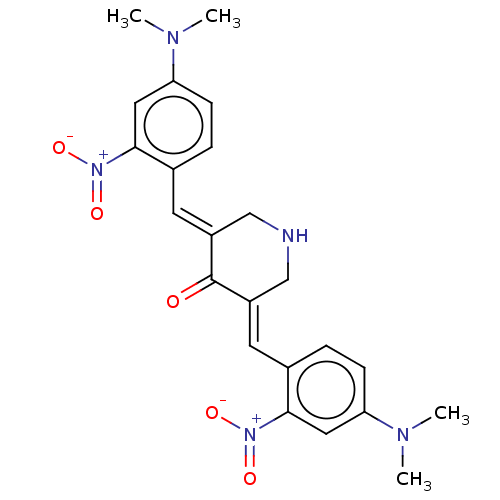 (CHEMBL3309948) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50045847 (CHEMBL3309947) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50021470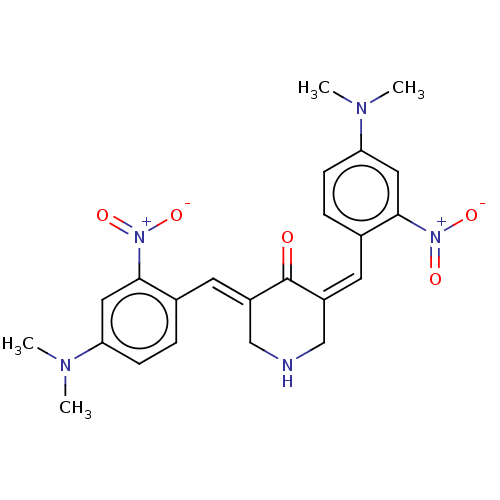 (CHEMBL3289936) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) after 5 mins by Ellman's method | Eur J Med Chem 83: 355-65 (2014) Article DOI: 10.1016/j.ejmech.2014.06.034 BindingDB Entry DOI: 10.7270/Q2862J10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM203833 (2-(2-Bromo-3,4,5-trimethoxy-benzylidene)-4-methyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM203860 (2-(2-Bromo-3,4,5-trimethoxy-benzylidene)-7-nitro-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50161796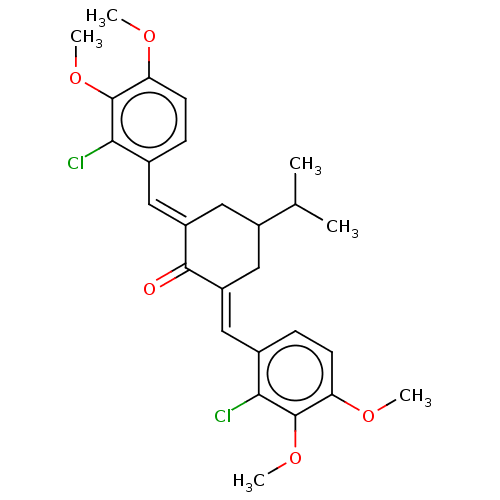 (CHEMBL3787250) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for 10 mi... | Bioorg Med Chem 24: 2352-9 (2016) Article DOI: 10.1016/j.bmc.2016.04.015 BindingDB Entry DOI: 10.7270/Q2S46TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203833 (2-(2-Bromo-3,4,5-trimethoxy-benzylidene)-4-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50021447 (CHEMBL3289926) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) after 5 mins by Ellman's method | Eur J Med Chem 83: 355-65 (2014) Article DOI: 10.1016/j.ejmech.2014.06.034 BindingDB Entry DOI: 10.7270/Q2862J10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM153297 (3-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-5-(4,7- dime...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz | Assay Description 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 (ovine) or COX-2(human recombinant), which was preincubated for 10 min in a water bath ... | Chem Biol Drug Des 85: 729-42 (2015) Article DOI: 10.1111/cbdd.12457 BindingDB Entry DOI: 10.7270/Q26W98TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203860 (2-(2-Bromo-3,4,5-trimethoxy-benzylidene)-7-nitro-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50021418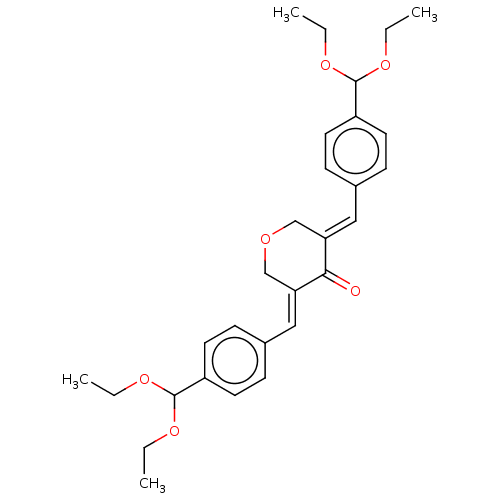 (CHEMBL3289925) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM153294 (3-Benzofuran-2-yl-5-(2,3-dimethoxy-naphthalen-1-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz | Assay Description 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 (ovine) or COX-2(human recombinant), which was preincubated for 10 min in a water bath ... | Chem Biol Drug Des 85: 729-42 (2015) Article DOI: 10.1111/cbdd.12457 BindingDB Entry DOI: 10.7270/Q26W98TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50252091 (CHEMBL4102284) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) assembly preincubated with enzyme followed by GTP addition measured after 20 mins by spectrophotometric analysis | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50251945 (CHEMBL4100075) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of His6-tagged recombinant EGFR cytoplasmic domain (unknown origin) (645 to 1186 residues) expressed in baculovirus infected Sf-9 cells pr... | Eur J Med Chem 135: 34-48 (2017) Article DOI: 10.1016/j.ejmech.2017.04.025 BindingDB Entry DOI: 10.7270/Q2H41TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM153289 (3-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-5-(4-dimethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz | Assay Description 100 mM Tris澦Cl buffer, pH 8.0 containing 1 然 heme and COX-1 (ovine) or COX-2(human recombinant), which was preincubated for 10 min in a water bath ... | Chem Biol Drug Des 85: 729-42 (2015) Article DOI: 10.1111/cbdd.12457 BindingDB Entry DOI: 10.7270/Q26W98TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50021467 (CHEMBL3289933) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 417 total ) | Next | Last >> |