 Found 302 hits with Last Name = 'cermak' and Initial = 'n'
Found 302 hits with Last Name = 'cermak' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Group 10 secretory phospholipase A2
(Mus musculus) | BDBM50263002
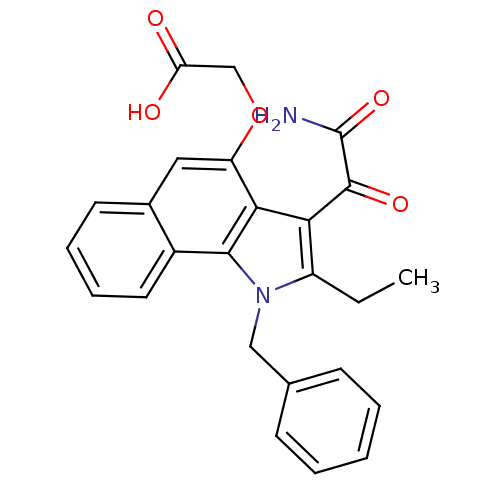
((2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-6,7-benz...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H22N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-12H,2,13-14H2,1H3,(H2,26,31)(H,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50262842
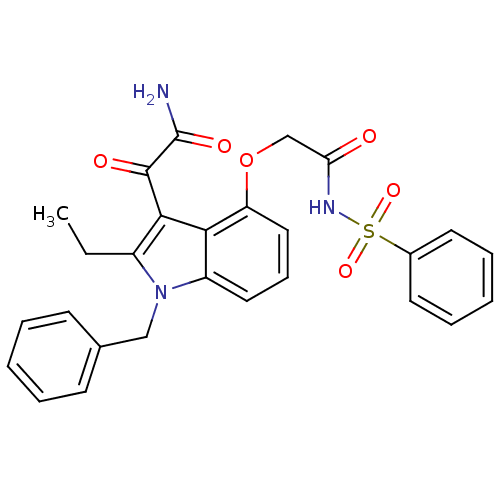
(Benzenesulfonyl-2-(3-(2-amino-2-oxoacetyl)-1-benzy...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C27H25N3O6S/c1-2-20-25(26(32)27(28)33)24-21(30(20)16-18-10-5-3-6-11-18)14-9-15-22(24)36-17-23(31)29-37(34,35)19-12-7-4-8-13-19/h3-15H,2,16-17H2,1H3,(H2,28,33)(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2E phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50262998
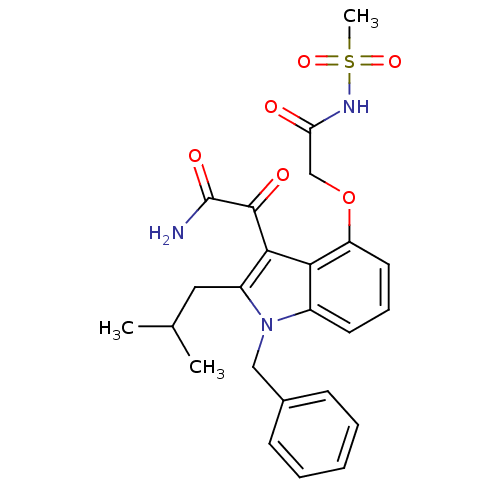
(CHEMBL477548 | mesyl-2-(3-(2-amino-2-oxoacetyl)-1-...)Show SMILES CC(C)Cc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(C)(=O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C24H27N3O6S/c1-15(2)12-18-22(23(29)24(25)30)21-17(27(18)13-16-8-5-4-6-9-16)10-7-11-19(21)33-14-20(28)26-34(3,31)32/h4-11,15H,12-14H2,1-3H3,(H2,25,30)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IID secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50263002

((2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-6,7-benz...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H22N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-12H,2,13-14H2,1H3,(H2,26,31)(H,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2D phospholipase A2 by [3H]oleic acid-labeled Escherichia coli membrane assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50263002

((2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-6,7-benz...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H22N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-12H,2,13-14H2,1H3,(H2,26,31)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2E phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50055391

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-ind...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC(C)C(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-19(20(25)21(23)26)18-16(24(15)12-14-8-5-4-6-9-14)10-7-11-17(18)29-13(2)22(27)28/h4-11,13H,3,12H2,1-2H3,(H2,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2E phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50053137

((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-ethyl-indol...)Show SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C27H24N2O5/c1-2-19-21(15-18-11-6-7-12-20(18)17-9-4-3-5-10-17)29-14-8-13-22(34-16-23(30)31)25(29)24(19)26(32)27(28)33/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2E phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Oryctolagus cuniculus) | BDBM50256011
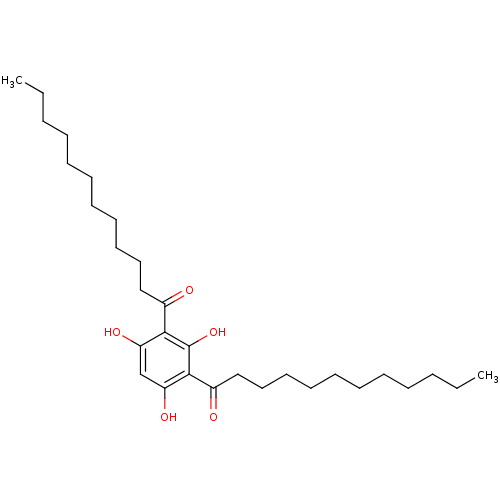
(1,1'-(2,4,6-trihydroxy-1,3-phenylene)didodecan-1-o...)Show SMILES CCCCCCCCCCCC(=O)c1c(O)cc(O)c(C(=O)CCCCCCCCCCC)c1O Show InChI InChI=1S/C30H50O5/c1-3-5-7-9-11-13-15-17-19-21-24(31)28-26(33)23-27(34)29(30(28)35)25(32)22-20-18-16-14-12-10-8-6-4-2/h23,33-35H,3-22H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of rabbit sPLA2 group 2A |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50262804
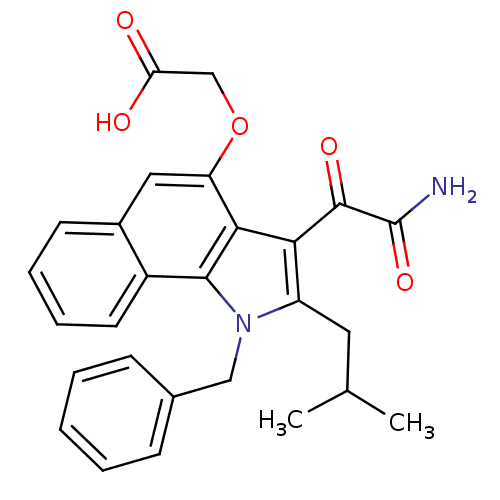
(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-isobutyl-1H-...)Show SMILES CC(C)Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C27H26N2O5/c1-16(2)12-20-23(26(32)27(28)33)24-21(34-15-22(30)31)13-18-10-6-7-11-19(18)25(24)29(20)14-17-8-4-3-5-9-17/h3-11,13,16H,12,14-15H2,1-2H3,(H2,28,33)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50262952

(CHEMBL514656 | o-tolyl-2-(3-(2-amino-2-oxoacetyl)-...)Show SMILES CC(C)Cc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3C)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C30H31N3O6S/c1-19(2)16-23-28(29(35)30(31)36)27-22(33(23)17-21-11-5-4-6-12-21)13-9-14-24(27)39-18-26(34)32-40(37,38)25-15-8-7-10-20(25)3/h4-15,19H,16-18H2,1-3H3,(H2,31,36)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Mus musculus) | BDBM50262842

(Benzenesulfonyl-2-(3-(2-amino-2-oxoacetyl)-1-benzy...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C27H25N3O6S/c1-2-20-25(26(32)27(28)33)24-21(30(20)16-18-10-5-3-6-11-18)14-9-15-22(24)36-17-23(31)29-37(34,35)19-12-7-4-8-13-19/h3-15H,2,16-17H2,1H3,(H2,28,33)(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2E phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50263003
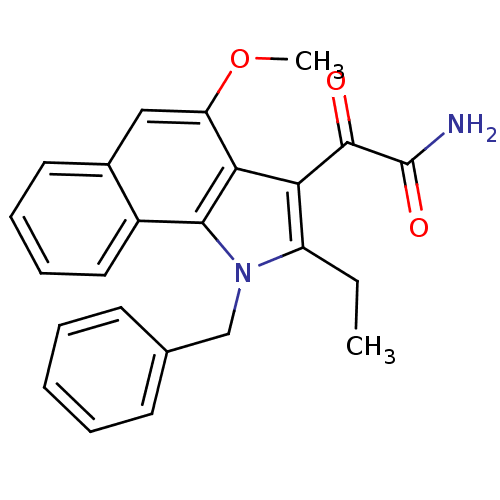
(2-(2-ethyl-3-(o-phenylbenzyl)-8-(2-oxopropoxy)indo...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C24H22N2O3/c1-3-18-20(23(27)24(25)28)21-19(29-2)13-16-11-7-8-12-17(16)22(21)26(18)14-15-9-5-4-6-10-15/h4-13H,3,14H2,1-2H3,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2A phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50262950

(2-Chloro-Benzenesulfonyl-2-(3-(2-amino-2-oxoacetyl...)Show SMILES CC(C)Cc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3Cl)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C29H28ClN3O6S/c1-18(2)15-22-27(28(35)29(31)36)26-21(33(22)16-19-9-4-3-5-10-19)12-8-13-23(26)39-17-25(34)32-40(37,38)24-14-7-6-11-20(24)30/h3-14,18H,15-17H2,1-2H3,(H2,31,36)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50262842

(Benzenesulfonyl-2-(3-(2-amino-2-oxoacetyl)-1-benzy...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C27H25N3O6S/c1-2-20-25(26(32)27(28)33)24-21(30(20)16-18-10-5-3-6-11-18)14-9-15-22(24)36-17-23(31)29-37(34,35)19-12-7-4-8-13-19/h3-15H,2,16-17H2,1H3,(H2,28,33)(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50263000
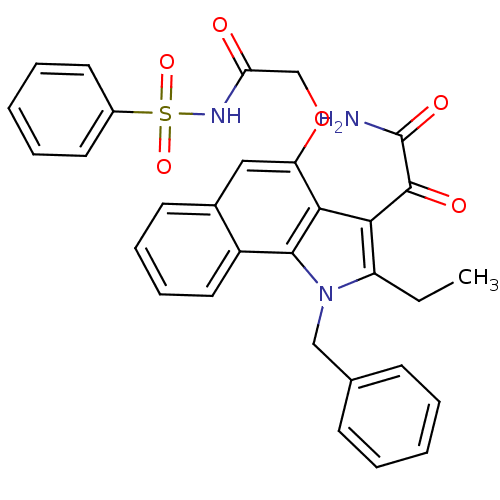
(2-(1-benzyl-2-ethyl-1H-6,7-benzoindol-4-yloxy)-N-(...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C31H27N3O6S/c1-2-24-27(30(36)31(32)37)28-25(40-19-26(35)33-41(38,39)22-14-7-4-8-15-22)17-21-13-9-10-16-23(21)29(28)34(24)18-20-11-5-3-6-12-20/h3-17H,2,18-19H2,1H3,(H2,32,37)(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2E phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Mus musculus) | BDBM50263002

((2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-6,7-benz...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H22N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-12H,2,13-14H2,1H3,(H2,26,31)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2E phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50263002

((2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-6,7-benz...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H22N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-12H,2,13-14H2,1H3,(H2,26,31)(H,28,29) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50263003

(2-(2-ethyl-3-(o-phenylbenzyl)-8-(2-oxopropoxy)indo...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C24H22N2O3/c1-3-18-20(23(27)24(25)28)21-19(29-2)13-16-11-7-8-12-17(16)22(21)26(18)14-15-9-5-4-6-10-15/h4-13H,3,14H2,1-2H3,(H2,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2E phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Mus musculus) | BDBM50055391

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-ind...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC(C)C(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-19(20(25)21(23)26)18-16(24(15)12-14-8-5-4-6-9-14)10-7-11-17(18)29-13(2)22(27)28/h4-11,13H,3,12H2,1-2H3,(H2,23,26)(H,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50263002

((2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-6,7-benz...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H22N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-12H,2,13-14H2,1H3,(H2,26,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50262843
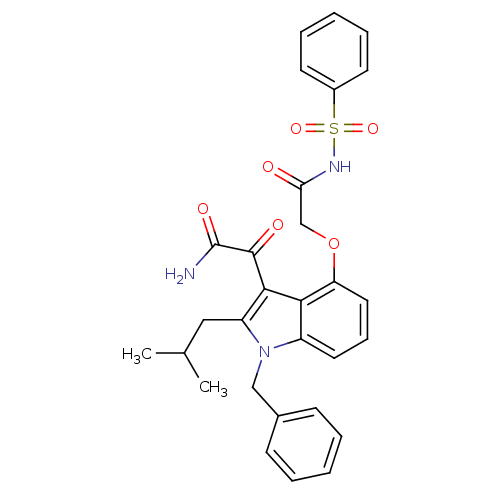
(Benzenesulfonyl-2-(3-(2-amino-2-oxoacetyl)-1-benzy...)Show SMILES CC(C)Cc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C29H29N3O6S/c1-19(2)16-23-27(28(34)29(30)35)26-22(32(23)17-20-10-5-3-6-11-20)14-9-15-24(26)38-18-25(33)31-39(36,37)21-12-7-4-8-13-21/h3-15,19H,16-18H2,1-2H3,(H2,30,35)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50055391

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-ind...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC(C)C(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-19(20(25)21(23)26)18-16(24(15)12-14-8-5-4-6-9-14)10-7-11-17(18)29-13(2)22(27)28/h4-11,13H,3,12H2,1-2H3,(H2,23,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Mus musculus) | BDBM50262842

(Benzenesulfonyl-2-(3-(2-amino-2-oxoacetyl)-1-benzy...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C27H25N3O6S/c1-2-20-25(26(32)27(28)33)24-21(30(20)16-18-10-5-3-6-11-18)14-9-15-22(24)36-17-23(31)29-37(34,35)19-12-7-4-8-13-19/h3-15H,2,16-17H2,1H3,(H2,28,33)(H,29,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Mus musculus) | BDBM50274336

(1-{3-Dodecanoyl-2,4,6-trihydroxy-5-[7-hydroxy-2-(4...)Show SMILES CCCCCCCCCCCC(=O)c1c(O)c(C2CC(Oc3cc(O)ccc23)c2ccc(O)cc2)c(O)c(C(=O)CCCCCCCCCCC)c1O Show InChI InChI=1S/C45H62O8/c1-3-5-7-9-11-13-15-17-19-21-36(48)41-43(50)40(44(51)42(45(41)52)37(49)22-20-18-16-14-12-10-8-6-4-2)35-30-38(31-23-25-32(46)26-24-31)53-39-29-33(47)27-28-34(35)39/h23-29,35,38,46-47,50-52H,3-22,30H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse sPLA2 group 2A |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50263052
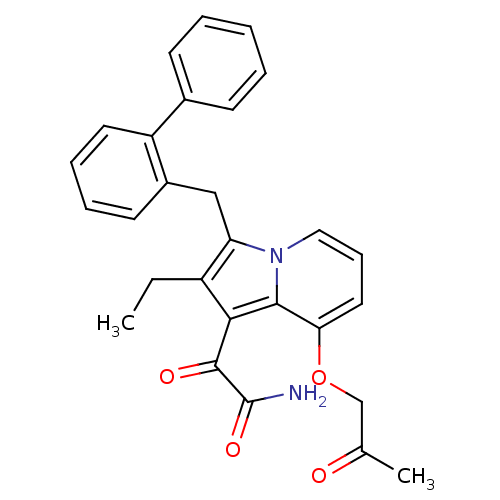
(2-[3-Biphenyl-2-ylmethyl-2-ethyl-8-(2-oxo-propoxy)...)Show SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(C)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C28H26N2O4/c1-3-21-23(16-20-12-7-8-13-22(20)19-10-5-4-6-11-19)30-15-9-14-24(34-17-18(2)31)26(30)25(21)27(32)28(29)33/h4-15H,3,16-17H2,1-2H3,(H2,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2A phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Mus musculus) | BDBM50263002

((2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-6,7-benz...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H22N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-12H,2,13-14H2,1H3,(H2,26,31)(H,28,29) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2A phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50263001
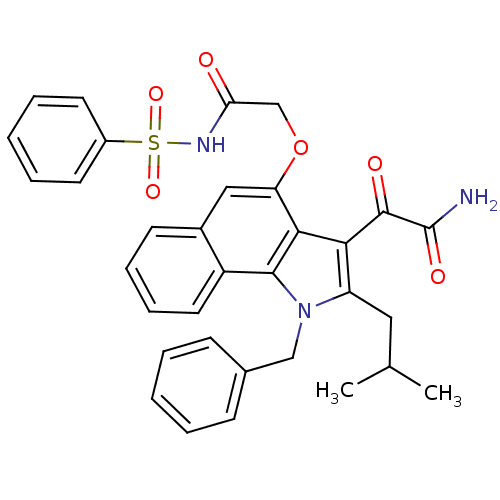
(2-(1-benzyl-2-isobutyl-1H-6,7-benzoindol-4-yloxy)-...)Show SMILES CC(C)Cc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C33H31N3O6S/c1-21(2)17-26-29(32(38)33(34)39)30-27(42-20-28(37)35-43(40,41)24-14-7-4-8-15-24)18-23-13-9-10-16-25(23)31(30)36(26)19-22-11-5-3-6-12-22/h3-16,18,21H,17,19-20H2,1-2H3,(H2,34,39)(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50262999

(CHEMBL477549 | trifluoromesyl-2-(3-(2-amino-2-oxoa...)Show SMILES CC(C)Cc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)C(F)(F)F)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C24H24F3N3O6S/c1-14(2)11-17-21(22(32)23(28)33)20-16(30(17)12-15-7-4-3-5-8-15)9-6-10-18(20)36-13-19(31)29-37(34,35)24(25,26)27/h3-10,14H,11-13H2,1-2H3,(H2,28,33)(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Mus musculus) | BDBM50263003

(2-(2-ethyl-3-(o-phenylbenzyl)-8-(2-oxopropoxy)indo...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C24H22N2O3/c1-3-18-20(23(27)24(25)28)21-19(29-2)13-16-11-7-8-12-17(16)22(21)26(18)14-15-9-5-4-6-10-15/h4-13H,3,14H2,1-2H3,(H2,25,28) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2A phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50263053
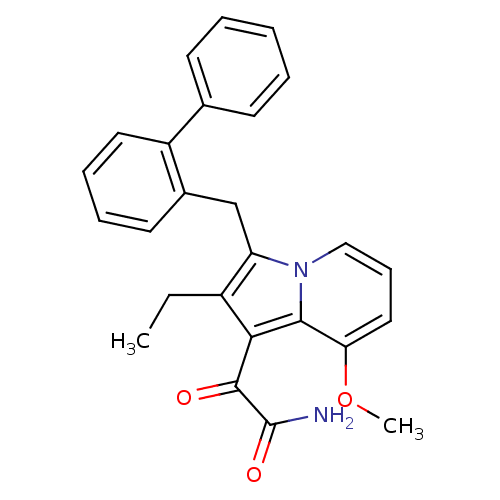
(2-(3-Biphenyl-2-ylmethyl-2-ethyl-8-methoxy-indoliz...)Show SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OC)c2c1C(=O)C(N)=O Show InChI InChI=1S/C26H24N2O3/c1-3-19-21(16-18-12-7-8-13-20(18)17-10-5-4-6-11-17)28-15-9-14-22(31-2)24(28)23(19)25(29)26(27)30/h4-15H,3,16H2,1-2H3,(H2,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2A phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50263002

((2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-6,7-benz...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H22N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-12H,2,13-14H2,1H3,(H2,26,31)(H,28,29) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Mus musculus) | BDBM50263000

(2-(1-benzyl-2-ethyl-1H-6,7-benzoindol-4-yloxy)-N-(...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C31H27N3O6S/c1-2-24-27(30(36)31(32)37)28-25(40-19-26(35)33-41(38,39)22-14-7-4-8-15-22)17-21-13-9-10-16-23(21)29(28)34(24)18-20-11-5-3-6-12-20/h3-17H,2,18-19H2,1H3,(H2,32,37)(H,33,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IID secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50263000

(2-(1-benzyl-2-ethyl-1H-6,7-benzoindol-4-yloxy)-N-(...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C31H27N3O6S/c1-2-24-27(30(36)31(32)37)28-25(40-19-26(35)33-41(38,39)22-14-7-4-8-15-22)17-21-13-9-10-16-23(21)29(28)34(24)18-20-11-5-3-6-12-20/h3-17H,2,18-19H2,1H3,(H2,32,37)(H,33,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2D phospholipase A2 by [3H]oleic acid-labeled Escherichia coli membrane assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Mus musculus) | BDBM50053137

((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-ethyl-indol...)Show SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C27H24N2O5/c1-2-19-21(15-18-11-6-7-12-20(18)17-9-4-3-5-10-17)29-14-8-13-22(34-16-23(30)31)25(29)24(19)26(32)27(28)33/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2E phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50263002

((2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-6,7-benz...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H22N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-12H,2,13-14H2,1H3,(H2,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2A phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50055391

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-ind...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC(C)C(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-19(20(25)21(23)26)18-16(24(15)12-14-8-5-4-6-9-14)10-7-11-17(18)29-13(2)22(27)28/h4-11,13H,3,12H2,1-2H3,(H2,23,26)(H,27,28) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Mus musculus) | BDBM50263000

(2-(1-benzyl-2-ethyl-1H-6,7-benzoindol-4-yloxy)-N-(...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C31H27N3O6S/c1-2-24-27(30(36)31(32)37)28-25(40-19-26(35)33-41(38,39)22-14-7-4-8-15-22)17-21-13-9-10-16-23(21)29(28)34(24)18-20-11-5-3-6-12-20/h3-17H,2,18-19H2,1H3,(H2,32,37)(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2E phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IIF secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50263002

((2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-6,7-benz...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H22N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-12H,2,13-14H2,1H3,(H2,26,31)(H,28,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2F phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50263053

(2-(3-Biphenyl-2-ylmethyl-2-ethyl-8-methoxy-indoliz...)Show SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OC)c2c1C(=O)C(N)=O Show InChI InChI=1S/C26H24N2O3/c1-3-19-21(16-18-12-7-8-13-20(18)17-10-5-4-6-11-17)28-15-9-14-22(31-2)24(28)23(19)25(29)26(27)30/h4-15H,3,16H2,1-2H3,(H2,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2E phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50262844
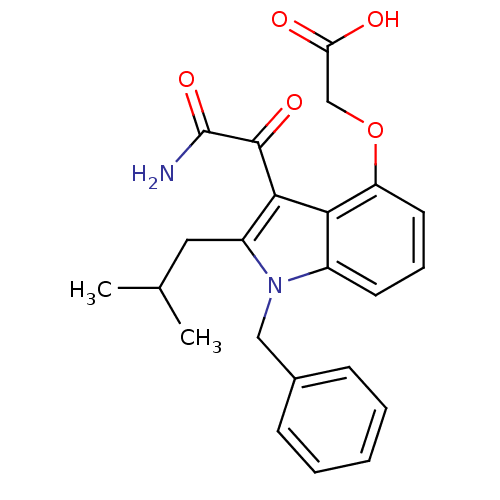
(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-isobutyl-1H-...)Show SMILES CC(C)Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C23H24N2O5/c1-14(2)11-17-21(22(28)23(24)29)20-16(9-6-10-18(20)30-13-19(26)27)25(17)12-15-7-4-3-5-8-15/h3-10,14H,11-13H2,1-2H3,(H2,24,29)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2X phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2E phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50055391

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-ind...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC(C)C(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-19(20(25)21(23)26)18-16(24(15)12-14-8-5-4-6-9-14)10-7-11-17(18)29-13(2)22(27)28/h4-11,13H,3,12H2,1-2H3,(H2,23,26)(H,27,28) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Group IID secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2D phospholipase A2 by [3H]oleic acid-labeled Escherichia coli membrane assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Mus musculus) | BDBM50055391

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-ind...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC(C)C(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-19(20(25)21(23)26)18-16(24(15)12-14-8-5-4-6-9-14)10-7-11-17(18)29-13(2)22(27)28/h4-11,13H,3,12H2,1-2H3,(H2,23,26)(H,27,28) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2A phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50262842

(Benzenesulfonyl-2-(3-(2-amino-2-oxoacetyl)-1-benzy...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C27H25N3O6S/c1-2-20-25(26(32)27(28)33)24-21(30(20)16-18-10-5-3-6-11-18)14-9-15-22(24)36-17-23(31)29-37(34,35)19-12-7-4-8-13-19/h3-15H,2,16-17H2,1H3,(H2,28,33)(H,29,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Mus musculus) | BDBM50262842

(Benzenesulfonyl-2-(3-(2-amino-2-oxoacetyl)-1-benzy...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C27H25N3O6S/c1-2-20-25(26(32)27(28)33)24-21(30(20)16-18-10-5-3-6-11-18)14-9-15-22(24)36-17-23(31)29-37(34,35)19-12-7-4-8-13-19/h3-15H,2,16-17H2,1H3,(H2,28,33)(H,29,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2A phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50053137

((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-ethyl-indol...)Show SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C27H24N2O5/c1-2-19-21(15-18-11-6-7-12-20(18)17-9-4-3-5-10-17)29-14-8-13-22(34-16-23(30)31)25(29)24(19)26(32)27(28)33/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2A phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Mus musculus) | BDBM50274337
(1-(3-Dodecanoyl-2,4,6-trihydroxy-5-methyl-phenyl)-...)Show SMILES CCCCCCCCCCCC(=O)c1c(O)c(C)c(O)c(C(=O)CCCCCCCCCCC)c1O Show InChI InChI=1S/C31H52O5/c1-4-6-8-10-12-14-16-18-20-22-25(32)27-29(34)24(3)30(35)28(31(27)36)26(33)23-21-19-17-15-13-11-9-7-5-2/h34-36H,4-23H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse sPLA2 group 2A |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Rattus norvegicus) | BDBM50274443
((+ +)-1-{3-Dodecanoyl-2,4,6-trihydroxy-5-[(2R,4R)-...)Show SMILES CCCCCCCCCCCC(=O)c1c(O)c([C@@H]2C[C@@H](Oc3cc(O)ccc23)c2ccc(O)cc2)c(O)c(C(=O)CCCCCCCCCCC)c1O |r| Show InChI InChI=1S/C45H62O8/c1-3-5-7-9-11-13-15-17-19-21-36(48)41-43(50)40(44(51)42(45(41)52)37(49)22-20-18-16-14-12-10-8-6-4-2)35-30-38(31-23-25-32(46)26-24-31)53-39-29-33(47)27-28-34(35)39/h23-29,35,38,46-47,50-52H,3-22,30H2,1-2H3/t35-,38-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of rat sPLA2 group 2A |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Mus musculus) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2A phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data