 Found 31 hits with Last Name = 'doti' and Initial = 'n'
Found 31 hits with Last Name = 'doti' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582780
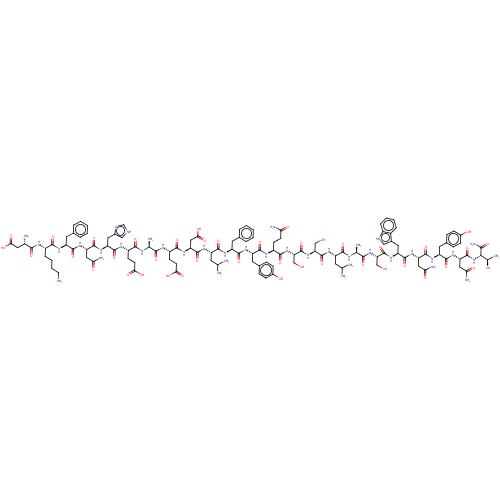
(CHEMBL5091821)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 expressed in HEK293T cells using FMZ as substrate by NanoLuc luciferase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582782
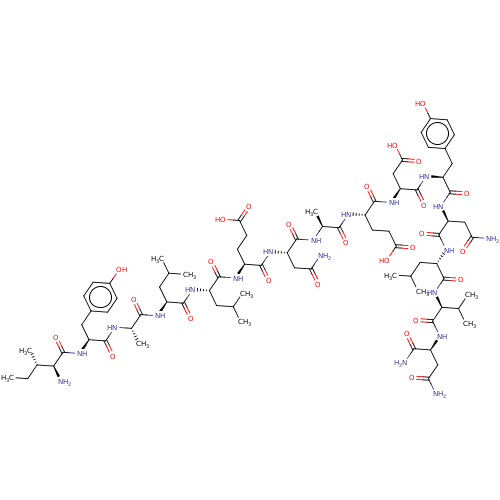
(CHEMBL5088125)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 expressed in HEK293T cells using FMZ as substrate by NanoLuc luciferase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582783
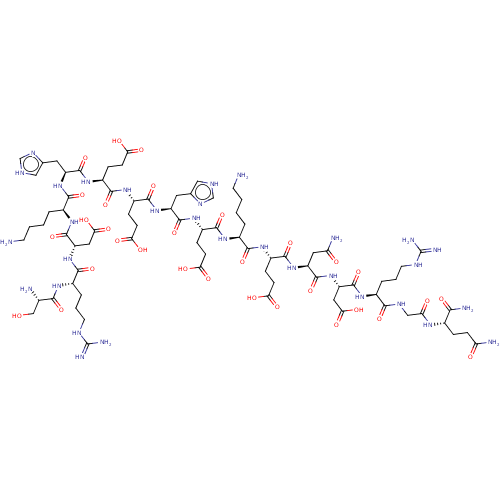
(CHEMBL5093610)Show SMILES NCCCC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 expressed in HEK293T cells using FMZ as substrate by NanoLuc luciferase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582778

(CHEMBL5081268)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 expressed in HEK293T cells using FMZ as substrate by NanoLuc luciferase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582779
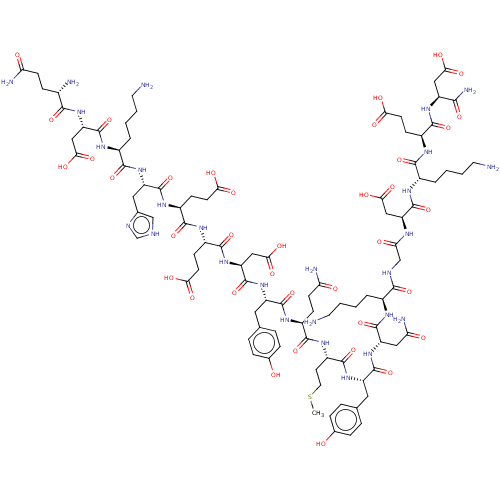
(CHEMBL5081573)Show SMILES CSCC[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 expressed in HEK293T cells using FMZ as substrate by NanoLuc luciferase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582781
(CHEMBL5078837)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(N)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 expressed in HEK293T cells using FMZ as substrate by NanoLuc luciferase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582782

(CHEMBL5088125)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 by ITC assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50559137

(CHEMBL4785828)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 by ITC assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582783

(CHEMBL5093610)Show SMILES NCCCC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 by ITC assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Trans-sialidase
(Trypanosoma cruzi) | BDBM84698
(Quinolinone derivative, 9)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1C(\C(=C/c2ccc(O)c(O)c2)C(=O)c2ccccc12)c1ccc(O)c(O)c1 Show InChI InChI=1S/C29H23NO7S/c1-17-6-10-20(11-7-17)38(36,37)30-23-5-3-2-4-21(23)29(35)22(14-18-8-12-24(31)26(33)15-18)28(30)19-9-13-25(32)27(34)16-19/h2-16,28,31-34H,1H3/b22-14+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sao Paulo
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trans-sialidase using Trifluoromethylumbelliferyl alpha-sialoside as substrate by UV/visible spectrophotometer |
Bioorg Med Chem 25: 6049-6059 (2017)
Article DOI: 10.1016/j.bmc.2017.09.042
BindingDB Entry DOI: 10.7270/Q21Z4700 |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50582780
(CHEMBL5091821)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant SARS-COV2 S-RBD incubated for 5 mins by microscale thermophoresis analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50582781

(CHEMBL5078837)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(N)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 541 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant SARS-COV2 S-RBD incubated for 5 mins by microscale thermophoresis analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50582782

(CHEMBL5088125)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant SARS-COV2 S-RBD incubated for 5 mins by microscale thermophoresis analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50582783

(CHEMBL5093610)Show SMILES NCCCC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant SARS-COV2 S-RBD incubated for 5 mins by microscale thermophoresis analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50582784

(CHEMBL5094981)Show SMILES C=O.CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC(C)C)[C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 spike protein mediated infection of human ACE2 expressing cells |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50582785
(CHEMBL5070871)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](C)N)[C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 spike protein mediated infection of human ACE2 expressing cells |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50582786
(CHEMBL5094988)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](C)N)[C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 spike protein mediated infection of human ACE2 expressing cells |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50582787
(CHEMBL5088895)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](N)CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 spike protein mediated infection of human ACE2 expressing cells |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50582787

(CHEMBL5088895)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](N)CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity of SARS-COV2 S-RBD by BLI assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50582786

(CHEMBL5094988)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](C)N)[C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity of SARS-COV2 S-RBD by BLI assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50582779

(CHEMBL5081573)Show SMILES CSCC[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant SARS-COV2 S-RBD incubated for 5 mins by microscale thermophoresis analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50582778

(CHEMBL5081268)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(N)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant SARS-COV2 S-RBD incubated for 5 mins by microscale thermophoresis analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50587149
(CHEMBL5082292)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC1=O)C(C)C)C(N)=O)NC(C)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to His6-tagged CypA (unknown origin) expressed in Escherichia coli BL21 (DE3) incubation at 1 hr by multimode plate reader |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00777
BindingDB Entry DOI: 10.7270/Q2BZ69ZX |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50587148

(CHEMBL5089595)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC1=O)C(C)C)C(N)=O)NC(C)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to His6-tagged CypA (unknown origin) expressed in Escherichia coli BL21 (DE3) incubation at 1 hr by multimode plate reader |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00777
BindingDB Entry DOI: 10.7270/Q2BZ69ZX |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50587147

(CHEMBL5094458)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(C)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CS)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to His6-tagged CypA (unknown origin) expressed in Escherichia coli BL21 (DE3) incubation at 1 hr by multimode plate reader |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00777
BindingDB Entry DOI: 10.7270/Q2BZ69ZX |
More data for this
Ligand-Target Pair | |
Galectin-3
(Homo sapiens (Human)) | BDBM50451465
(CHEMBL4216668)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OCc2cn(nn2)-c2ccc(cc2)S(=O)(=O)Nc2cc(OC)nc(OC)n2)[C@H]1O |r| Show InChI InChI=1S/C22H28N6O10S/c1-34-17-8-16(23-22(24-17)36-3)26-39(32,33)14-6-4-13(5-7-14)28-9-12(25-27-28)11-37-20-18(30)15(10-29)38-21(35-2)19(20)31/h4-9,15,18-21,29-31H,10-11H2,1-3H3,(H,23,24,26)/t15-,18+,19-,20+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a |
University of Sao Paulo
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human galectin-3 expressed in Escherichia coli BL21 after 3 hrs |
Bioorg Med Chem 25: 6049-6059 (2017)
Article DOI: 10.1016/j.bmc.2017.09.042
BindingDB Entry DOI: 10.7270/Q21Z4700 |
More data for this
Ligand-Target Pair | |
Galectin-3
(Homo sapiens (Human)) | BDBM50451464
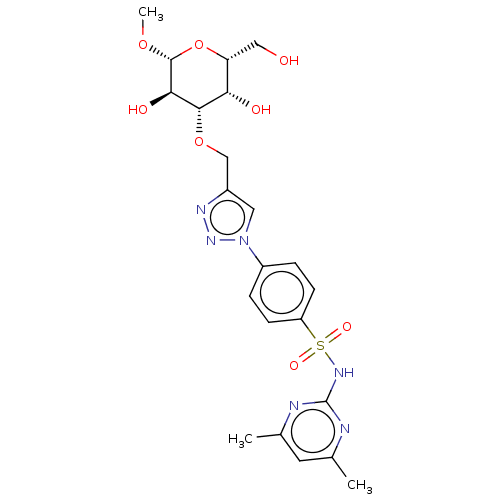
(CHEMBL4211695)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OCc2cn(nn2)-c2ccc(cc2)S(=O)(=O)Nc2nc(C)cc(C)n2)[C@H]1O |r| Show InChI InChI=1S/C22H28N6O8S/c1-12-8-13(2)24-22(23-12)26-37(32,33)16-6-4-15(5-7-16)28-9-14(25-27-28)11-35-20-18(30)17(10-29)36-21(34-3)19(20)31/h4-9,17-21,29-31H,10-11H2,1-3H3,(H,23,24,26)/t17-,18+,19-,20+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.77E+4 | n/a | n/a | n/a | n/a |
University of Sao Paulo
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human galectin-3 expressed in Escherichia coli BL21 after 3 hrs |
Bioorg Med Chem 25: 6049-6059 (2017)
Article DOI: 10.1016/j.bmc.2017.09.042
BindingDB Entry DOI: 10.7270/Q21Z4700 |
More data for this
Ligand-Target Pair | |
Galectin-3
(Homo sapiens (Human)) | BDBM50451463
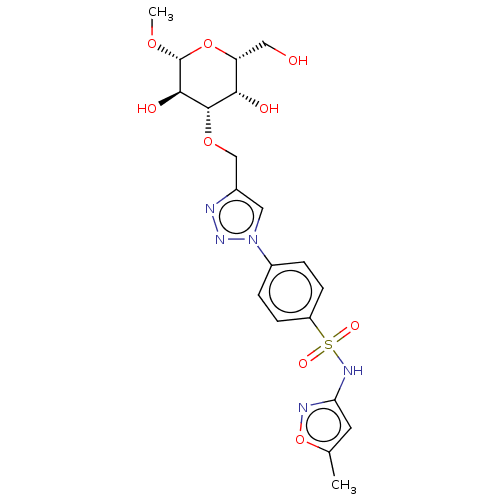
(CHEMBL4212225)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OCc2cn(nn2)-c2ccc(cc2)S(=O)(=O)Nc2cc(C)on2)[C@H]1O |r| Show InChI InChI=1S/C20H25N5O9S/c1-11-7-16(22-34-11)23-35(29,30)14-5-3-13(4-6-14)25-8-12(21-24-25)10-32-19-17(27)15(9-26)33-20(31-2)18(19)28/h3-8,15,17-20,26-28H,9-10H2,1-2H3,(H,22,23)/t15-,17+,18-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a |
University of Sao Paulo
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human galectin-3 expressed in Escherichia coli BL21 after 3 hrs |
Bioorg Med Chem 25: 6049-6059 (2017)
Article DOI: 10.1016/j.bmc.2017.09.042
BindingDB Entry DOI: 10.7270/Q21Z4700 |
More data for this
Ligand-Target Pair | |
Spike glycoprotein
(2019-nCoV) | BDBM50582787

(CHEMBL5088895)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](N)CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to biotinylated recombinant SARS-COV2 S-RBD by SPR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Galectin-3
(Homo sapiens (Human)) | BDBM50451461
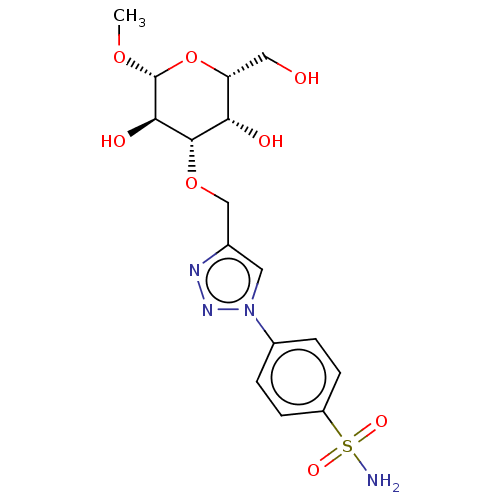
(CHEMBL4207873)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OCc2cn(nn2)-c2ccc(cc2)S(N)(=O)=O)[C@H]1O |r| Show InChI InChI=1S/C16H22N4O8S/c1-26-16-14(23)15(13(22)12(7-21)28-16)27-8-9-6-20(19-18-9)10-2-4-11(5-3-10)29(17,24)25/h2-6,12-16,21-23H,7-8H2,1H3,(H2,17,24,25)/t12-,13+,14-,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.07E+4 | n/a | n/a | n/a | n/a |
University of Sao Paulo
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human galectin-3 expressed in Escherichia coli BL21 after 3 hrs |
Bioorg Med Chem 25: 6049-6059 (2017)
Article DOI: 10.1016/j.bmc.2017.09.042
BindingDB Entry DOI: 10.7270/Q21Z4700 |
More data for this
Ligand-Target Pair | |
Galectin-3
(Homo sapiens (Human)) | BDBM50451462
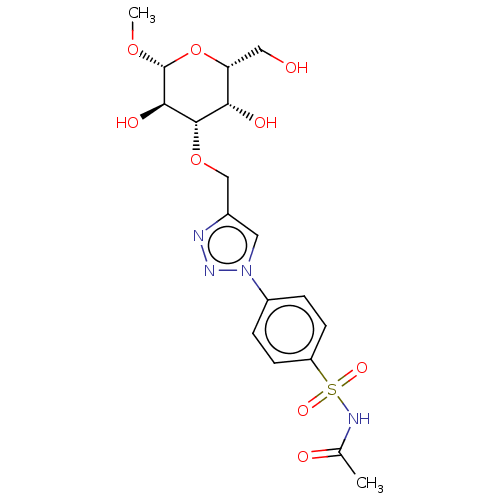
(CHEMBL4207339)Show SMILES CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OCc2cn(nn2)-c2ccc(cc2)S(=O)(=O)NC(C)=O)[C@H]1O |r| Show InChI InChI=1S/C18H24N4O9S/c1-10(24)20-32(27,28)13-5-3-12(4-6-13)22-7-11(19-21-22)9-30-17-15(25)14(8-23)31-18(29-2)16(17)26/h3-7,14-18,23,25-26H,8-9H2,1-2H3,(H,20,24)/t14-,15+,16-,17+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.05E+5 | n/a | n/a | n/a | n/a |
University of Sao Paulo
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human galectin-3 expressed in Escherichia coli BL21 after 3 hrs |
Bioorg Med Chem 25: 6049-6059 (2017)
Article DOI: 10.1016/j.bmc.2017.09.042
BindingDB Entry DOI: 10.7270/Q21Z4700 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data