 Found 5278 hits with Last Name = 'erez' and Initial = 'n'
Found 5278 hits with Last Name = 'erez' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85097
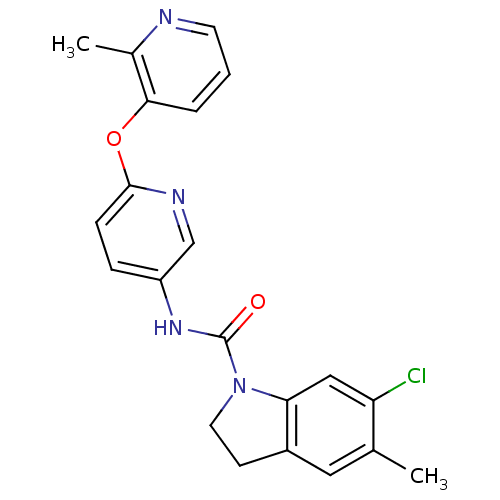
(CAS_181632-25-7 | CHEMBL14563 | SB 242084)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(Oc4cccnc4C)nc3)c2cc1Cl Show InChI InChI=1S/C21H19ClN4O2/c1-13-10-15-7-9-26(18(15)11-17(13)22)21(27)25-16-5-6-20(24-12-16)28-19-4-3-8-23-14(19)2/h3-6,8,10-12H,7,9H2,1-2H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Dominican College
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human 5-HT2C expressed in human U2OS cells by pathhunter beta-arrestin assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127358
BindingDB Entry DOI: 10.7270/Q26113XJ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dominican College
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human 5-HT2C expressed in human U2OS cells by pathhunter beta-arrestin assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127358
BindingDB Entry DOI: 10.7270/Q26113XJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50113332
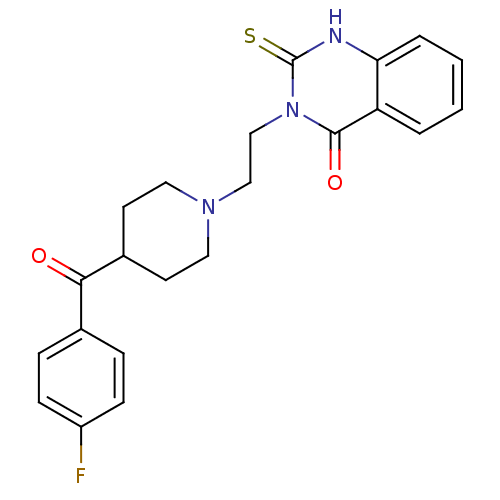
(3-(2-(4-(4-fluorobenzoyl)piperidin-1-yl)ethyl)-2-t...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=S)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O2S/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dominican College
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human 5-HT2A expressed in human U2OS cells by pathhunter beta-arrestin assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127358
BindingDB Entry DOI: 10.7270/Q26113XJ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50097721
(CHEMBL1879790 | EN300-11843)Show InChI InChI=1S/C29H38FN5O3S/c1-4-6-7-8-9-10-15-33(3)26(36)20-35-19-24(16-23-17-31-28(38)34(5-2)18-23)27(37)32-29(35)39-21-22-11-13-25(30)14-12-22/h11-14,17-19H,4-10,15-16,20-21H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496902

(CVD-0018409 | PF-07321332 | US11351149, Example 13...)Show SMILES CC(C)(C)[C@H](NC(=O)C(F)(F)F)C(=O)N1C[C@H]2[C@@H]([C@H]1C(=O)N[C@@H](C[C@@H]1CCNC1=O)C#N)C2(C)C Show InChI InChI=1S/C23H32F3N5O4/c1-21(2,3)16(30-20(35)23(24,25)26)19(34)31-10-13-14(22(13,4)5)15(31)18(33)29-12(9-27)8-11-6-7-28-17(11)32/h11-16H,6-8,10H2,1-5H3,(H,28,32)(H,29,33)(H,30,35)/t11-,12-,13-,14-,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM536747

(US11247985, Table 3.51)Show SMILES CC(C)N1CCN(CC1)C1(CNC2CCN(CC2)c2cccc(c2)-c2cc3cc(ccc3[nH]2)C#N)CCC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50468106

(CHEMBL4280801 | US11247985, Table 3.49)Show SMILES CN1CCN(CC1)C1(CNC2CCN(CC2)c2cccc(c2)-c2cc3cc(F)ccc3[nH]2)CCC1 Show InChI InChI=1S/C29H38FN5/c1-33-14-16-35(17-15-33)29(10-3-11-29)21-31-25-8-12-34(13-9-25)26-5-2-4-22(19-26)28-20-23-18-24(30)6-7-27(23)32-28/h2,4-7,18-20,25,31-32H,3,8-17,21H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496478

(LOR-NOR-30067bb9-11)Show InChI InChI=1S/C16H9BrN2O2/c17-13-6-2-5-12-14(13)19(16(21)15(12)20)9-11-4-1-3-10(7-11)8-18/h1-7H,9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Israel Institution of Biological Research
| Assay Description
The assay was performed according to the published procedure. Briefly, compounds were seeded into assay-ready plates (Greiner 384PP, cat# 781280) usi... |
bioRxiv 2021: (2021)
BindingDB Entry DOI: 10.7270/Q2MS3WV7 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM627791
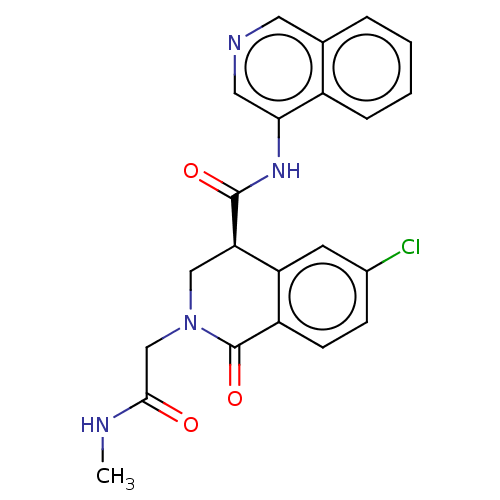
(CVD-0019230)Show SMILES CNC(=O)CN1C[C@@H](C(=O)Nc2cncc3ccccc23)c2cc(Cl)ccc2C1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM495599

(CVD-0016335 | STE-KUL-d79e3d6a-3)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](NC(=O)CCCC#C)C(C)(C)C)C(=O)NN(CCC(N)=O)C(=O)CCl Show InChI InChI=1S/C23H38ClN5O5/c1-7-8-9-10-18(31)27-20(23(4,5)6)22(34)26-16(13-15(2)3)21(33)28-29(19(32)14-24)12-11-17(25)30/h1,15-16,20H,8-14H2,2-6H3,(H2,25,30)(H,26,34)(H,27,31)(H,28,33)/t16-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM495599

(CVD-0016335 | STE-KUL-d79e3d6a-3)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](NC(=O)CCCC#C)C(C)(C)C)C(=O)NN(CCC(N)=O)C(=O)CCl Show InChI InChI=1S/C23H38ClN5O5/c1-7-8-9-10-18(31)27-20(23(4,5)6)22(34)26-16(13-15(2)3)21(33)28-29(19(32)14-24)12-11-17(25)30/h1,15-16,20H,8-14H2,2-6H3,(H2,25,30)(H,26,34)(H,27,31)(H,28,33)/t16-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Israel Institution of Biological Research
| Assay Description
Compounds were seeded into assay-ready plates (Greiner 384 low volume, cat 784900) using an Echo 555 acoustic dispenser, and DMSO was back-filled for... |
bioRxiv 2021: (2021)
BindingDB Entry DOI: 10.7270/Q2MS3WV7 |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM536749
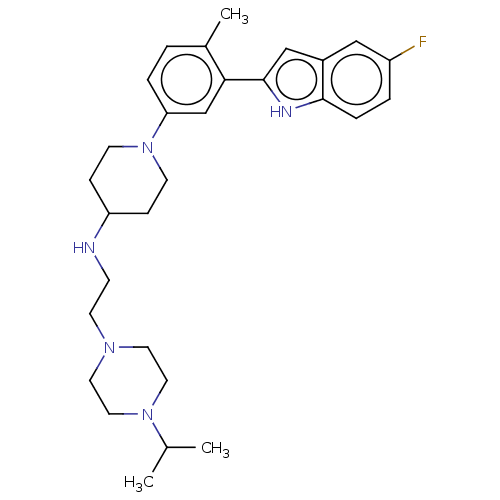
(US11247985, Table 3.53)Show SMILES CC(C)N1CCN(CCNC2CCN(CC2)c2ccc(C)c(c2)-c2cc3cc(F)ccc3[nH]2)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496258
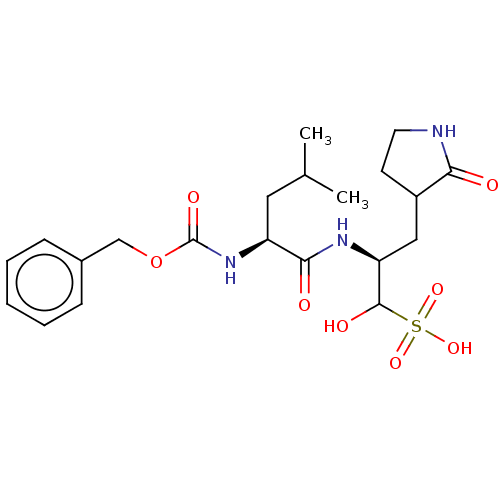
(CVD-0013146 | JOH-MSK-46727e7b-1)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S(O)(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Israel Institution of Biological Research
| Assay Description
The assay was performed according to the published procedure. Briefly, compounds were seeded into assay-ready plates (Greiner 384PP, cat# 781280) usi... |
bioRxiv 2021: (2021)
BindingDB Entry DOI: 10.7270/Q2MS3WV7 |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM536753

(US11247985, Table 3.57)Show SMILES CN1CCN(CC1)C1(CNC2CCN(CC2)c2cccc(c2)-c2cc3cc(ccc3[nH]2)C#N)CCC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50514013
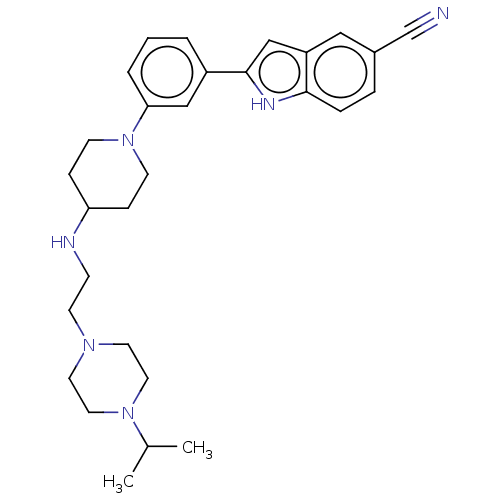
(CHEMBL4445673 | US11247985, Table 3.55)Show SMILES CC(C)N1CCN(CCNC2CCN(CC2)c2cccc(c2)-c2cc3cc(ccc3[nH]2)C#N)CC1 Show InChI InChI=1S/C29H38N6/c1-22(2)34-16-14-33(15-17-34)13-10-31-26-8-11-35(12-9-26)27-5-3-4-24(19-27)29-20-25-18-23(21-30)6-7-28(25)32-29/h3-7,18-20,22,26,31-32H,8-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50468108
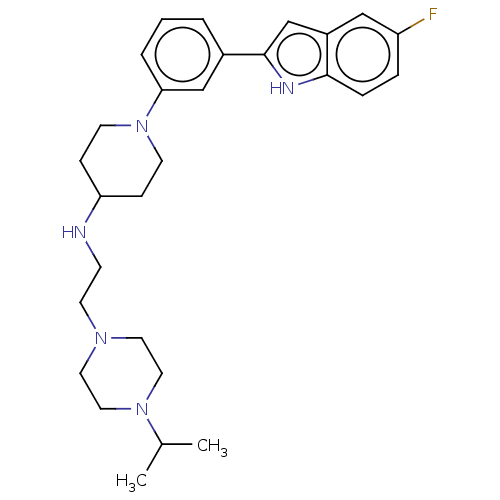
(CHEMBL4280125 | US11247985, Table 3.59)Show SMILES CC(C)N1CCN(CCNC2CCN(CC2)c2cccc(c2)-c2cc3cc(F)ccc3[nH]2)CC1 Show InChI InChI=1S/C28H38FN5/c1-21(2)33-16-14-32(15-17-33)13-10-30-25-8-11-34(12-9-25)26-5-3-4-22(19-26)28-20-23-18-24(29)6-7-27(23)31-28/h3-7,18-21,25,30-31H,8-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM627915
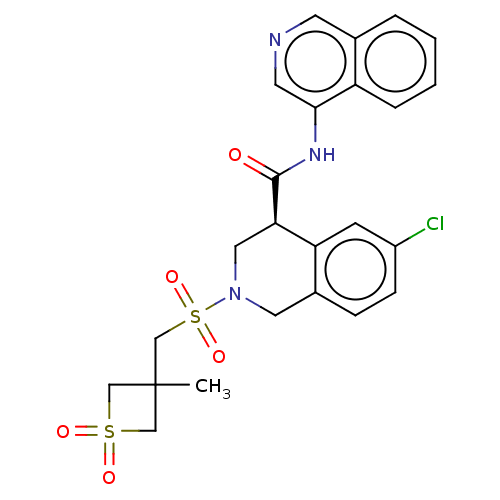
(CVD-0019273)Show SMILES CC1(CS(=O)(=O)N2C[C@@H](C(=O)Nc3cncc4ccccc34)c3cc(Cl)ccc3C2)CS(=O)(=O)C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Dominican College
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human 5-HT2A expressed in human U2OS cells by pathhunter beta-arrestin assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127358
BindingDB Entry DOI: 10.7270/Q26113XJ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM625991
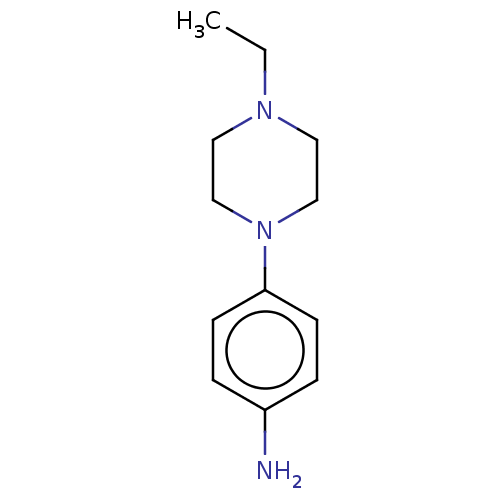
(EN300-11760) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496258

(CVD-0013146 | JOH-MSK-46727e7b-1)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CC1CCNC1=O)C(O)S(O)(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/t15?,16-,17-,20?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Israel Institution of Biological Research
| Assay Description
Compounds were seeded into assay-ready plates (Greiner 384 low volume, cat 784900) using an Echo 555 acoustic dispenser, and DMSO was back-filled for... |
bioRxiv 2021: (2021)
BindingDB Entry DOI: 10.7270/Q2MS3WV7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50544115

(Adatanserin hydrochloride | WY-50324 HCL)Show SMILES Cl.O=C(NCCN1CCN(CC1)c1ncccn1)C12CC3CC(CC(C3)C1)C2 |TLB:21:22:26:19.20.25,THB:21:20:26:27.22.23,23:22:19:26.24.25,23:24:19:27.22.21| Show InChI InChI=1S/C21H31N5O/c27-19(21-13-16-10-17(14-21)12-18(11-16)15-21)22-4-5-25-6-8-26(9-7-25)20-23-2-1-3-24-20/h1-3,16-18H,4-15H2,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Dominican College
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human 5-HT2A expressed in human U2OS cells by pathhunter beta-arrestin assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127358
BindingDB Entry DOI: 10.7270/Q26113XJ |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM626053
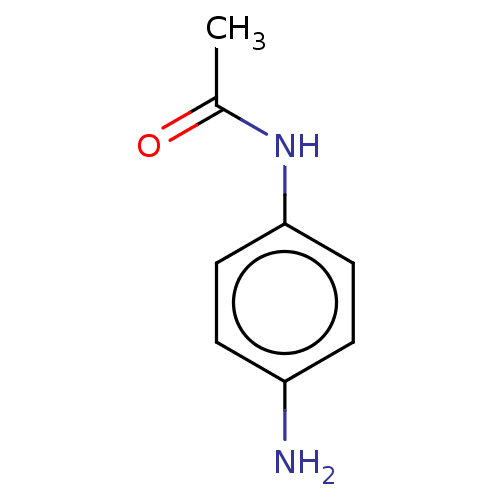
(EN300-17406) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50158905
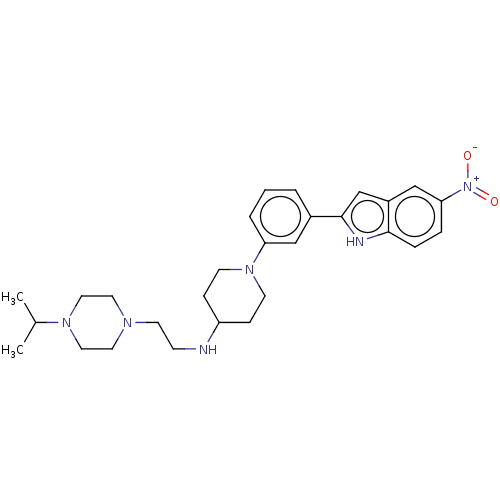
(CHEMBL3787674 | US11247985, Table 3.61)Show SMILES CC(C)N1CCN(CCNC2CCN(CC2)c2cccc(c2)-c2cc3cc(ccc3[nH]2)[N+]([O-])=O)CC1 Show InChI InChI=1S/C28H38N6O2/c1-21(2)32-16-14-31(15-17-32)13-10-29-24-8-11-33(12-9-24)25-5-3-4-22(18-25)28-20-23-19-26(34(35)36)6-7-27(23)30-28/h3-7,18-21,24,29-30H,8-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM495290
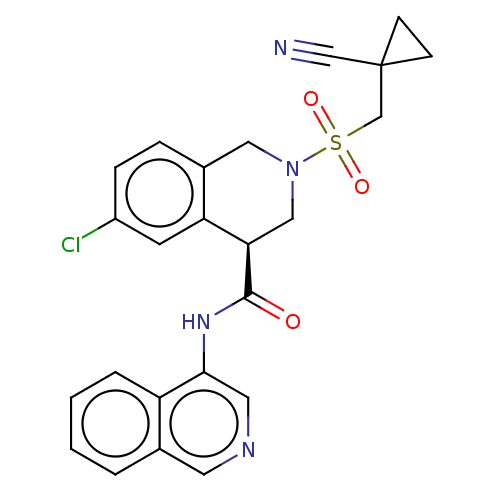
(CVD-0018692 | MAT-POS-e194df51-1)Show SMILES Clc1ccc2CN(C[C@@H](C(=O)Nc3cncc4ccccc34)c2c1)S(=O)(=O)CC1(CC1)C#N Show InChI InChI=1S/C24H21ClN4O3S/c25-18-6-5-17-12-29(33(31,32)15-24(14-26)7-8-24)13-21(20(17)9-18)23(30)28-22-11-27-10-16-3-1-2-4-19(16)22/h1-6,9-11,21H,7-8,12-13,15H2,(H,28,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Israel Institution of Biological Research
| Assay Description
Compounds were seeded into assay-ready plates (Greiner 384 low volume, cat 784900) using an Echo 555 acoustic dispenser, and DMSO was back-filled for... |
bioRxiv 2021: (2021)
BindingDB Entry DOI: 10.7270/Q2MS3WV7 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM495290

(CVD-0018692 | MAT-POS-e194df51-1)Show SMILES Clc1ccc2CN(C[C@@H](C(=O)Nc3cncc4ccccc34)c2c1)S(=O)(=O)CC1(CC1)C#N Show InChI InChI=1S/C24H21ClN4O3S/c25-18-6-5-17-12-29(33(31,32)15-24(14-26)7-8-24)13-21(20(17)9-18)23(30)28-22-11-27-10-16-3-1-2-4-19(16)22/h1-6,9-11,21H,7-8,12-13,15H2,(H,28,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM627860
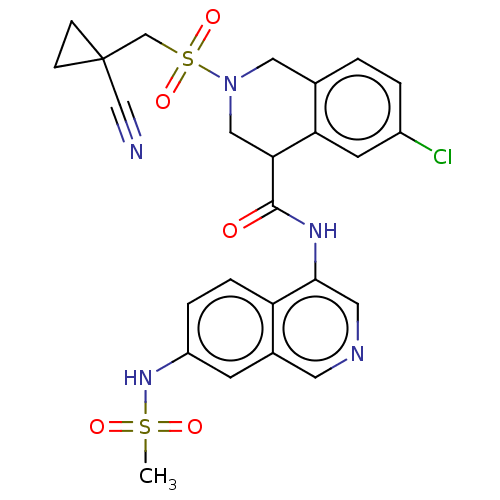
(CVD-0018385 | EDJ-MED-be9e6f63-3)Show SMILES CS(=O)(=O)Nc1ccc2c(NC(=O)C3CN(Cc4ccc(Cl)cc34)S(=O)(=O)CC3(CC3)C#N)cncc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM627860

(CVD-0018385 | EDJ-MED-be9e6f63-3)Show SMILES CS(=O)(=O)Nc1ccc2c(NC(=O)C3CN(Cc4ccc(Cl)cc34)S(=O)(=O)CC3(CC3)C#N)cncc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM625991

(EN300-11760) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50468112
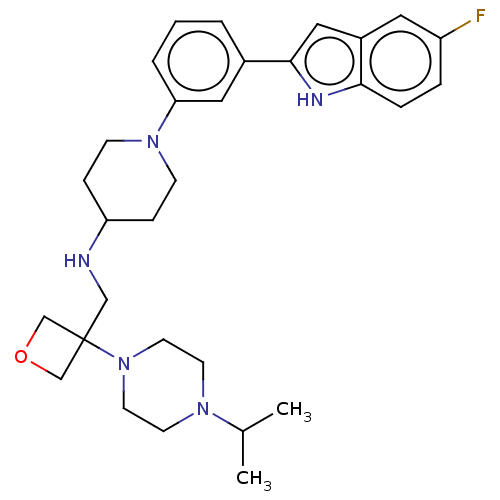
(CHEMBL4277404 | US11247985, Table 3.63)Show SMILES CC(C)N1CCN(CC1)C1(CNC2CCN(CC2)c2cccc(c2)-c2cc3cc(F)ccc3[nH]2)COC1 Show InChI InChI=1S/C30H40FN5O/c1-22(2)34-12-14-36(15-13-34)30(20-37-21-30)19-32-26-8-10-35(11-9-26)27-5-3-4-23(17-27)29-18-24-16-25(31)6-7-28(24)33-29/h3-7,16-18,22,26,32-33H,8-15,19-21H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM495803

(CVD-0015332 | MAT-POS-e69ad64a-2)Show SMILES Clc1ccc2OCC(C(=O)N(C(=O)C=C)c3cncc4ccccc34)c2c1 Show InChI InChI=1S/C21H15ClN2O3/c1-2-20(25)24(18-11-23-10-13-5-3-4-6-15(13)18)21(26)17-12-27-19-8-7-14(22)9-16(17)19/h2-11,17H,1,12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Israel Institution of Biological Research
| Assay Description
Compounds were seeded into assay-ready plates (Greiner 384 low volume, cat 784900) using an Echo 555 acoustic dispenser, and DMSO was back-filled for... |
bioRxiv 2021: (2021)
BindingDB Entry DOI: 10.7270/Q2MS3WV7 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM495803

(CVD-0015332 | MAT-POS-e69ad64a-2)Show SMILES Clc1ccc2OCC(C(=O)N(C(=O)C=C)c3cncc4ccccc34)c2c1 Show InChI InChI=1S/C21H15ClN2O3/c1-2-20(25)24(18-11-23-10-13-5-3-4-6-15(13)18)21(26)17-12-27-19-8-7-14(22)9-16(17)19/h2-11,17H,1,12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM627972
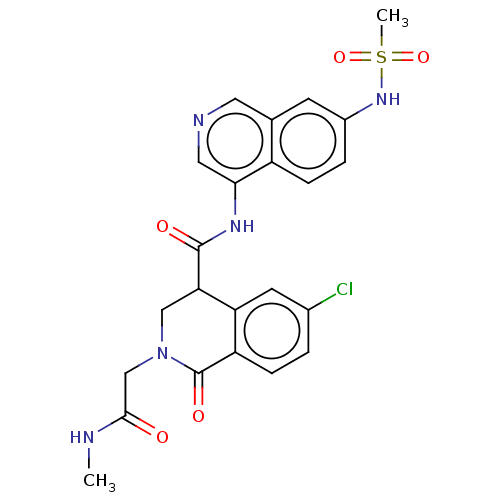
(CVD-0019478)Show SMILES CNC(=O)CN1CC(C(=O)Nc2cncc3cc(NS(C)(=O)=O)ccc23)c2cc(Cl)ccc2C1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM495598
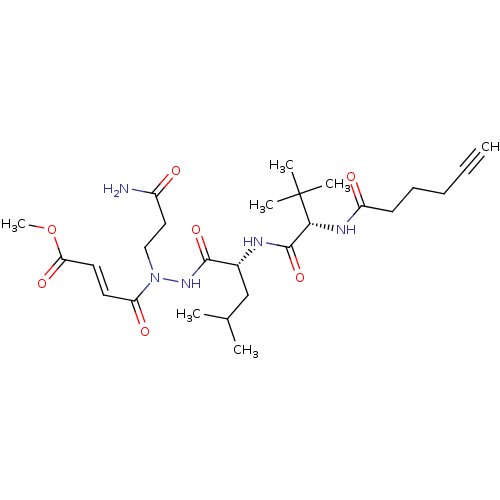
(CVD-0016334 | STE-KUL-d79e3d6a-2)Show SMILES COC(=O)\C=C\C(=O)N(CCC(N)=O)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCC#C)C(C)(C)C Show InChI InChI=1S/C26H41N5O7/c1-8-9-10-11-20(33)29-23(26(4,5)6)25(37)28-18(16-17(2)3)24(36)30-31(15-14-19(27)32)21(34)12-13-22(35)38-7/h1,12-13,17-18,23H,9-11,14-16H2,2-7H3,(H2,27,32)(H,28,37)(H,29,33)(H,30,36)/b13-12+/t18-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM495598

(CVD-0016334 | STE-KUL-d79e3d6a-2)Show SMILES COC(=O)\C=C\C(=O)N(CCC(N)=O)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)CCCC#C)C(C)(C)C Show InChI InChI=1S/C26H41N5O7/c1-8-9-10-11-20(33)29-23(26(4,5)6)25(37)28-18(16-17(2)3)24(36)30-31(15-14-19(27)32)21(34)12-13-22(35)38-7/h1,12-13,17-18,23H,9-11,14-16H2,2-7H3,(H2,27,32)(H,28,37)(H,29,33)(H,30,36)/b13-12+/t18-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Israel Institution of Biological Research
| Assay Description
Compounds were seeded into assay-ready plates (Greiner 384 low volume, cat 784900) using an Echo 555 acoustic dispenser, and DMSO was back-filled for... |
bioRxiv 2021: (2021)
BindingDB Entry DOI: 10.7270/Q2MS3WV7 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM626189

(EN300-60157) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM495783

(MAT-POS-f9802937-8)Show SMILES Clc1cc2NCC[C@@H](C(=O)Nc3cncc4ccccc34)c2cc1Cl Show InChI InChI=1S/C19H15Cl2N3O/c20-15-7-14-13(5-6-23-17(14)8-16(15)21)19(25)24-18-10-22-9-11-3-1-2-4-12(11)18/h1-4,7-10,13,23H,5-6H2,(H,24,25)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM495783

(MAT-POS-f9802937-8)Show SMILES Clc1cc2NCC[C@@H](C(=O)Nc3cncc4ccccc34)c2cc1Cl Show InChI InChI=1S/C19H15Cl2N3O/c20-15-7-14-13(5-6-23-17(14)8-16(15)21)19(25)24-18-10-22-9-11-3-1-2-4-12(11)18/h1-4,7-10,13,23H,5-6H2,(H,24,25)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM536761

(US11247985, Table 3.65 | US11247985, Table 3.78)Show SMILES CC(C)N1CCC(CC1)NC1CCN(CC1)c1cccc(c1)-c1cc2cc(ccc2[nH]1)C#N | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM495783

(MAT-POS-f9802937-8)Show SMILES Clc1cc2NCC[C@@H](C(=O)Nc3cncc4ccccc34)c2cc1Cl Show InChI InChI=1S/C19H15Cl2N3O/c20-15-7-14-13(5-6-23-17(14)8-16(15)21)19(25)24-18-10-22-9-11-3-1-2-4-12(11)18/h1-4,7-10,13,23H,5-6H2,(H,24,25)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Israel Institution of Biological Research
| Assay Description
Compounds were seeded into assay-ready plates (Greiner 384 low volume, cat 784900) using an Echo 555 acoustic dispenser, and DMSO was back-filled for... |
bioRxiv 2021: (2021)
BindingDB Entry DOI: 10.7270/Q2MS3WV7 |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50468110
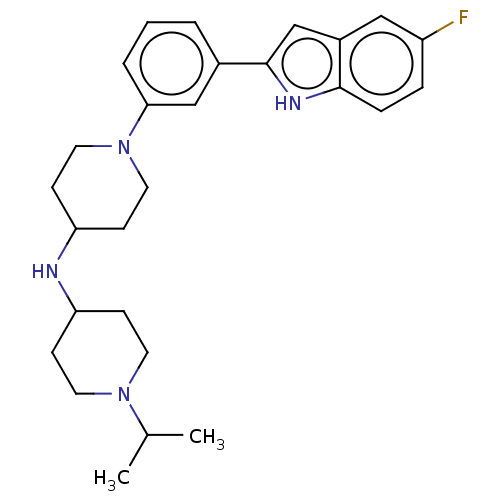
(CHEMBL4288865 | US11247985, Table 3.67)Show SMILES CC(C)N1CCC(CC1)NC1CCN(CC1)c1cccc(c1)-c1cc2cc(F)ccc2[nH]1 Show InChI InChI=1S/C27H35FN4/c1-19(2)31-12-8-23(9-13-31)29-24-10-14-32(15-11-24)25-5-3-4-20(17-25)27-18-21-16-22(28)6-7-26(21)30-27/h3-7,16-19,23-24,29-30H,8-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To optimize p97 inhibitors, the C-5 trifluoromethylated trifluoromethylated indole 12 was generated as a promising lead structure. In the ADP-Glo ass... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q509B |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM626053

(EN300-17406) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM626102
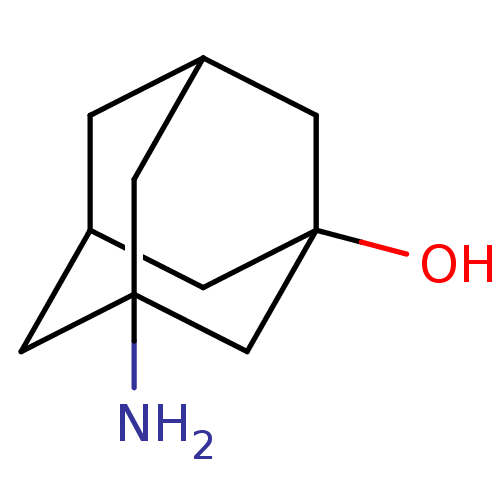
(EN300-213378)Show SMILES NC12CC3CC(C1)CC(O)(C3)C2 |TLB:4:3:11:5.6.7,4:5:11:2.3.10,THB:7:5:2:11.8.10,7:8:2:4.5.6| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM628026

(CVD-0019470)Show SMILES CCS(=O)(=O)Nc1ccc2c(NC(=O)C3CN(Cc4ccc(Cl)cc34)S(=O)(=O)CC3(CC3)C#N)cncc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM628051

(CVD-0019468)Show SMILES CS(=O)(=O)Nc1ccc2c(NC(=O)[C@@H]3CN(Cc4ccc(Cl)cc34)S(=O)(=O)CC3(CC3)C#N)cncc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM628060

(CVD-0019472)Show SMILES Clc1ccc2CN(CC(C(=O)Nc3cncc4cc(NS(=O)(=O)C5CC5)ccc34)c2c1)S(=O)(=O)CC1(CC1)C#N | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM429304
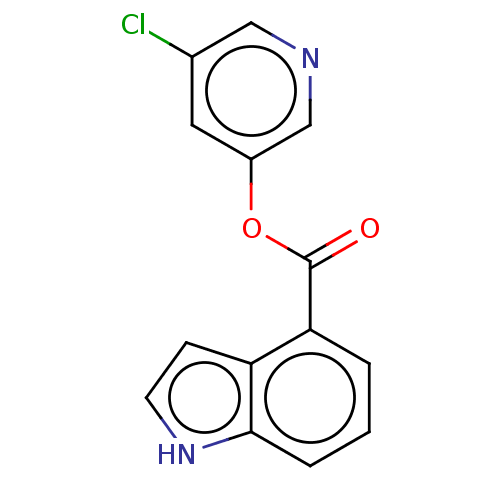
(CVD-0006354 | acs.jmedchem.1c00409_ST.426 | indole...)Show InChI InChI=1S/C14H9ClN2O2/c15-9-6-10(8-16-7-9)19-14(18)12-2-1-3-13-11(12)4-5-17-13/h1-8,17H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM628066
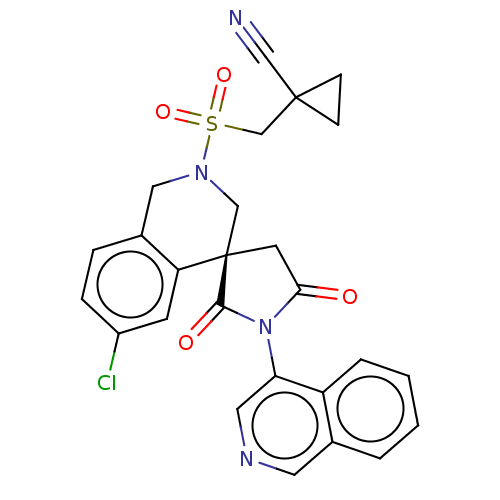
(CVD-0019645)Show SMILES Clc1ccc2CN(C[C@]3(CC(=O)N(C3=O)c3cncc4ccccc34)c2c1)S(=O)(=O)CC1(CC1)C#N | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM628069
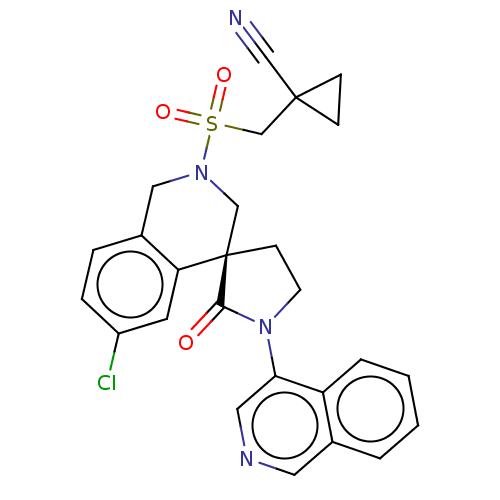
(CVD-0019750)Show SMILES Clc1ccc2CN(C[C@]3(CCN(C3=O)c3cncc4ccccc34)c2c1)S(=O)(=O)CC1(CC1)C#N | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM628149
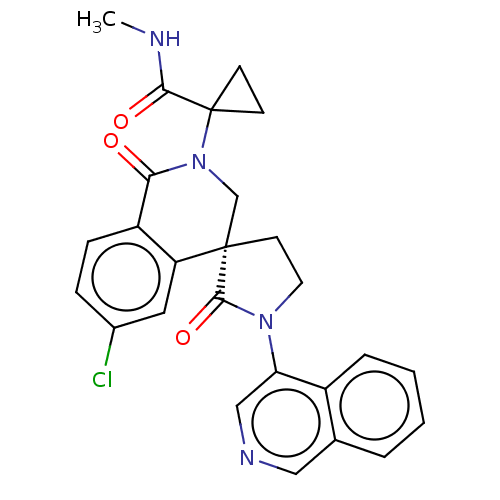
(CVD-0020128)Show SMILES CNC(=O)C1(CC1)N1C[C@@]2(CCN(C2=O)c2cncc3ccccc23)c2cc(Cl)ccc2C1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data