 Found 16 hits with Last Name = 'tan' and Initial = 'nh'
Found 16 hits with Last Name = 'tan' and Initial = 'nh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50237601
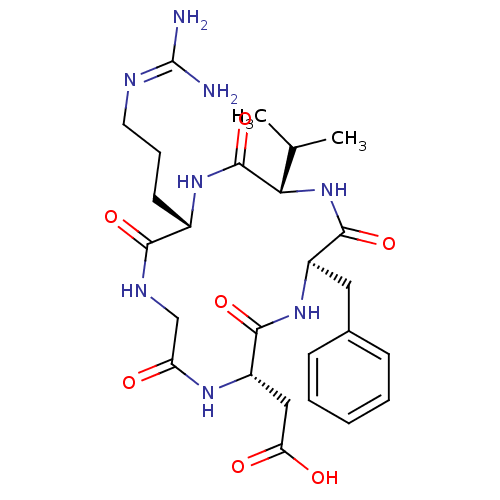
(CHEMBL411941 | CycloRGDfV | [(2S,5R,8S,11S)-5-Benz...)Show SMILES [#6]-[#6](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C26H38N8O7/c1-14(2)21-25(41)32-16(9-6-10-29-26(27)28)22(38)30-13-19(35)31-18(12-20(36)37)23(39)33-17(24(40)34-21)11-15-7-4-3-5-8-15/h3-5,7-8,14,16-18,21H,6,9-13H2,1-2H3,(H,30,38)(H,31,35)(H,32,41)(H,33,39)(H,34,40)(H,36,37)(H4,27,28,29)/t16-,17+,18-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.487 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-v-beta-3 integrin receptor by ELISA |
Bioorg Med Chem Lett 16: 6178-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.042
BindingDB Entry DOI: 10.7270/Q2C25069 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50359991
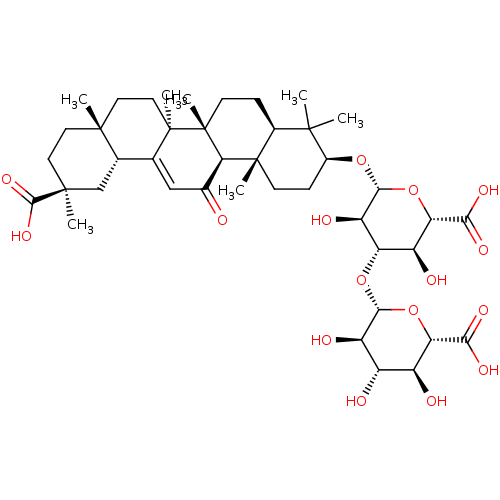
(CHEMBL1927953)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)O[C@@H]1O[C@@H]([C@@H](O)[C@H](O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)[C@H]1O)C(O)=O |r,t:18| Show InChI InChI=1S/C42H62O16/c1-37(2)21-8-11-42(7)31(20(43)16-18-19-17-39(4,36(53)54)13-12-38(19,3)14-15-41(18,42)6)40(21,5)10-9-22(37)55-35-27(48)28(26(47)30(58-35)33(51)52)56-34-25(46)23(44)24(45)29(57-34)32(49)50/h16,19,21-31,34-35,44-48H,8-15,17H2,1-7H3,(H,49,50)(H,51,52)(H,53,54)/t19-,21-,22-,23-,24-,25+,26-,27+,28-,29-,30-,31+,34+,35+,38+,39-,40-,41+,42+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Kunming Institute of Botany
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD2 expressed in human HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximity as... |
J Nat Prod 74: 2571-5 (2011)
Article DOI: 10.1021/np200755t
BindingDB Entry DOI: 10.7270/Q2P26ZJJ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063140
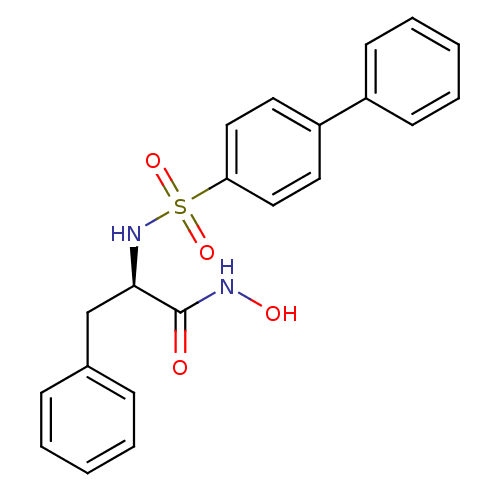
((2R)-[(4-biphenylylsulfonyl) amino]-N-hydroxy-3-ph...)Show SMILES ONC(=O)[C@@H](Cc1ccccc1)NS(=O)(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C21H20N2O4S/c24-21(22-25)20(15-16-7-3-1-4-8-16)23-28(26,27)19-13-11-18(12-14-19)17-9-5-2-6-10-17/h1-14,20,23,25H,15H2,(H,22,24)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.06 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 by quenched fluorescense assay |
Bioorg Med Chem Lett 16: 6178-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.042
BindingDB Entry DOI: 10.7270/Q2C25069 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50359991

(CHEMBL1927953)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)O[C@@H]1O[C@@H]([C@@H](O)[C@H](O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)[C@H]1O)C(O)=O |r,t:18| Show InChI InChI=1S/C42H62O16/c1-37(2)21-8-11-42(7)31(20(43)16-18-19-17-39(4,36(53)54)13-12-38(19,3)14-15-41(18,42)6)40(21,5)10-9-22(37)55-35-27(48)28(26(47)30(58-35)33(51)52)56-34-25(46)23(44)24(45)29(57-34)32(49)50/h16,19,21-31,34-35,44-48H,8-15,17H2,1-7H3,(H,49,50)(H,51,52)(H,53,54)/t19-,21-,22-,23-,24-,25+,26-,27+,28-,29-,30-,31+,34+,35+,38+,39-,40-,41+,42+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kunming Institute of Botany
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in human HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximity as... |
J Nat Prod 74: 2571-5 (2011)
Article DOI: 10.1021/np200755t
BindingDB Entry DOI: 10.7270/Q2P26ZJJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50359991

(CHEMBL1927953)Show SMILES CC1(C)[C@H](CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)O[C@@H]1O[C@@H]([C@@H](O)[C@H](O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)[C@H]1O)C(O)=O |r,t:18| Show InChI InChI=1S/C42H62O16/c1-37(2)21-8-11-42(7)31(20(43)16-18-19-17-39(4,36(53)54)13-12-38(19,3)14-15-41(18,42)6)40(21,5)10-9-22(37)55-35-27(48)28(26(47)30(58-35)33(51)52)56-34-25(46)23(44)24(45)29(57-34)32(49)50/h16,19,21-31,34-35,44-48H,8-15,17H2,1-7H3,(H,49,50)(H,51,52)(H,53,54)/t19-,21-,22-,23-,24-,25+,26-,27+,28-,29-,30-,31+,34+,35+,38+,39-,40-,41+,42+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kunming Institute of Botany
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in human HEK293 cells assessed as conversion of [3H]cortisone to [3H]cortisol by scintillation proximity as... |
J Nat Prod 74: 2571-5 (2011)
Article DOI: 10.1021/np200755t
BindingDB Entry DOI: 10.7270/Q2P26ZJJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50213272
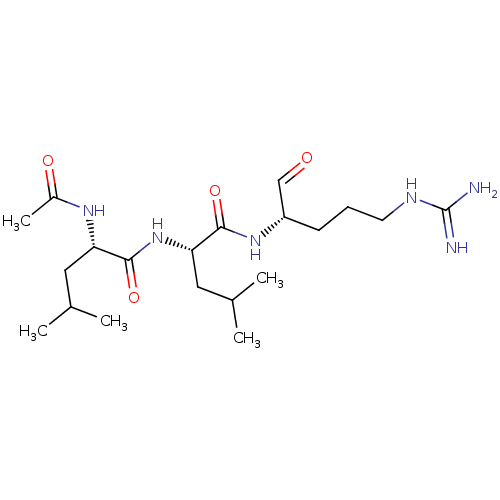
(CHEBI:6426 | Leupeptin)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C=O |r| Show InChI InChI=1S/C20H38N6O4/c1-12(2)9-16(24-14(5)28)19(30)26-17(10-13(3)4)18(29)25-15(11-27)7-6-8-23-20(21)22/h11-13,15-17H,6-10H2,1-5H3,(H,24,28)(H,25,29)(H,26,30)(H4,21,22,23)/t15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin K |
Bioorg Med Chem Lett 16: 6178-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.042
BindingDB Entry DOI: 10.7270/Q2C25069 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50323210
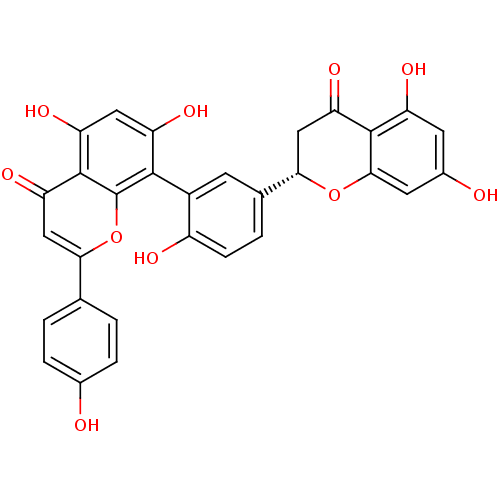
(2,3-dihydroamentoflavone | CHEMBL220741)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)[C@@H]3CC(=O)c4c(O)cc(O)cc4O3)c2o1 |wD:25.26,(3.82,-20.03,;3.83,-21.57,;5.16,-22.34,;5.17,-23.87,;3.83,-24.64,;2.5,-23.88,;2.49,-22.35,;3.83,-26.18,;5.17,-26.95,;5.17,-28.49,;6.5,-29.26,;3.84,-29.26,;3.85,-30.8,;5.18,-31.56,;2.51,-31.57,;1.18,-30.81,;-.15,-31.58,;1.18,-29.26,;-.15,-28.5,;-1.48,-29.27,;-2.82,-28.5,;-2.83,-26.97,;-1.5,-26.19,;-.16,-26.96,;1.17,-26.18,;-4.15,-29.27,;-4.15,-30.83,;-5.49,-31.6,;-5.49,-33.14,;-6.83,-30.84,;-8.17,-31.6,;-8.17,-33.14,;-9.51,-30.83,;-9.5,-29.29,;-10.84,-28.52,;-8.18,-28.52,;-6.84,-29.28,;-5.5,-28.5,;2.5,-28.49,;2.49,-26.96,)| Show InChI InChI=1S/C30H20O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-10,12,25,31-36H,11H2/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin K |
Bioorg Med Chem Lett 16: 6178-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.042
BindingDB Entry DOI: 10.7270/Q2C25069 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50323196
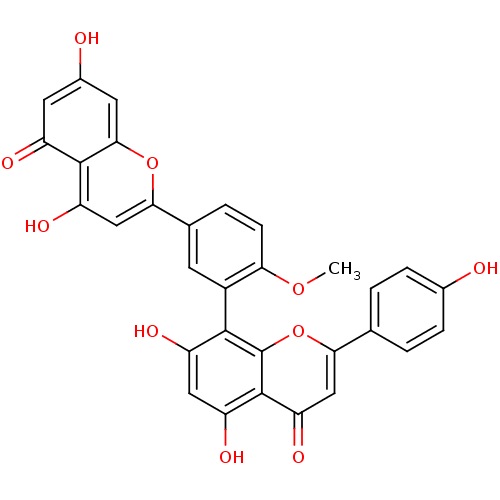
(4'-methylamentoflavone | CHEMBL378188 | bilobetin)Show SMILES COc1ccc(cc1-c1c(O)cc(O)c2c1oc(cc2=O)-c1ccc(O)cc1)-c1cc(O)c2c(cc(O)cc2=O)o1 |(3.27,-31.58,;3.27,-33.11,;1.94,-33.89,;.61,-33.11,;-.73,-33.89,;-.73,-35.42,;.61,-36.2,;1.94,-35.42,;3.27,-36.2,;4.6,-35.42,;4.6,-33.89,;5.93,-36.2,;5.93,-37.73,;7.27,-38.51,;4.6,-38.51,;3.27,-37.73,;1.94,-38.51,;1.94,-40.04,;3.27,-40.81,;4.6,-40.04,;5.93,-40.81,;.61,-40.81,;.61,-42.35,;-.73,-43.12,;-2.06,-42.35,;-3.39,-43.12,;-2.06,-40.81,;-.73,-40.04,;-2.06,-36.2,;-2.06,-37.73,;-3.39,-38.51,;-3.39,-40.04,;-4.73,-37.73,;-4.73,-36.2,;-6.06,-35.42,;-7.39,-36.2,;-8.72,-35.42,;-7.39,-37.73,;-6.06,-38.51,;-6.06,-40.04,;-3.39,-35.42,)| Show InChI InChI=1S/C31H20O10/c1-39-24-7-4-15(26-12-22(37)29-19(34)9-17(33)10-27(29)40-26)8-18(24)28-20(35)11-21(36)30-23(38)13-25(41-31(28)30)14-2-5-16(32)6-3-14/h2-13,32-33,35-37H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin K |
Bioorg Med Chem Lett 16: 6178-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.042
BindingDB Entry DOI: 10.7270/Q2C25069 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50323214
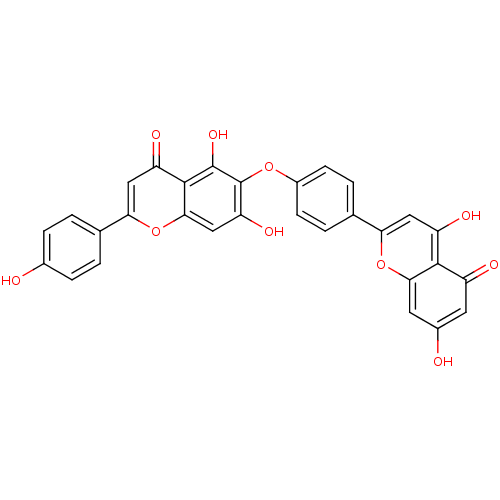
(6-[4-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-phenoxy...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)c(Oc3ccc(cc3)-c3cc(O)c4c(cc(O)cc4=O)o3)c(O)cc2o1 Show InChI InChI=1S/C30H18O10/c31-16-5-1-14(2-6-16)24-12-21(35)28-26(40-24)13-22(36)30(29(28)37)38-18-7-3-15(4-8-18)23-11-20(34)27-19(33)9-17(32)10-25(27)39-23/h1-13,31-32,34,36-37H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin K |
Bioorg Med Chem Lett 16: 6178-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.042
BindingDB Entry DOI: 10.7270/Q2C25069 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50323204

(7'',4'''-dimethylamentoflavone | CHEMBL374055)Show SMILES COc1ccc(cc1)-c1cc(=O)c2c(O)cc(OC)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(6.65,-6.92,;5.32,-7.68,;5.32,-9.22,;6.66,-9.99,;6.66,-11.53,;5.32,-12.3,;3.99,-11.54,;3.99,-10,;5.32,-13.84,;6.66,-14.6,;6.66,-16.14,;7.99,-16.91,;5.33,-16.91,;5.34,-18.45,;6.68,-19.22,;4.01,-19.23,;2.67,-18.46,;1.34,-19.23,;1.34,-20.77,;2.68,-16.92,;1.34,-16.15,;.01,-16.92,;-1.33,-16.15,;-1.33,-14.62,;-.01,-13.84,;1.34,-14.61,;2.67,-13.83,;-2.66,-16.92,;-2.66,-18.48,;-4,-19.26,;-4,-20.8,;-5.34,-18.49,;-5.35,-16.94,;-6.68,-16.17,;-8.01,-16.94,;-9.34,-16.17,;-8.01,-18.49,;-6.68,-19.26,;-6.68,-20.8,;-4.01,-16.15,;4,-16.15,;3.98,-14.61,)| Show InChI InChI=1S/C32H22O10/c1-39-18-6-3-15(4-7-18)25-13-23(37)31-24(38)14-27(40-2)29(32(31)42-25)19-9-16(5-8-20(19)34)26-12-22(36)30-21(35)10-17(33)11-28(30)41-26/h3-14,33-34,36,38H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin K |
Bioorg Med Chem Lett 16: 6178-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.042
BindingDB Entry DOI: 10.7270/Q2C25069 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin K |
Bioorg Med Chem Lett 16: 6178-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.042
BindingDB Entry DOI: 10.7270/Q2C25069 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50476091

(8,8''-Biskoenigine | CHEMBL434383)Show SMILES COc1cc2c([nH]c3c4C=CC(C)(C)Oc4c(C)cc23)c(c1O)-c1c(O)c(OC)cc2c1[nH]c1c3C=CC(C)(C)Oc3c(C)cc21 |c:9,40,(-.28,-.22,;-.29,-1.76,;1.05,-2.54,;2.38,-1.78,;3.7,-2.55,;3.69,-4.09,;5.15,-4.57,;6.06,-3.32,;7.59,-3.17,;8.48,-4.42,;10.01,-4.28,;10.65,-2.88,;12.05,-3.52,;11.63,-1.68,;9.77,-1.62,;8.24,-1.77,;7.33,-.51,;7.96,.9,;5.8,-.66,;5.16,-2.08,;2.37,-4.85,;1.04,-4.08,;-.29,-4.85,;2.28,-6.82,;3.61,-7.58,;4.94,-6.82,;3.6,-9.13,;4.93,-9.9,;4.93,-11.44,;2.27,-9.88,;.95,-9.11,;.96,-7.58,;-.5,-7.1,;-1.41,-8.34,;-2.94,-8.5,;-3.83,-7.25,;-5.36,-7.39,;-6.01,-8.78,;-7.4,-8.15,;-6.98,-9.98,;-5.12,-10.05,;-3.59,-9.9,;-2.68,-11.16,;-3.31,-12.56,;-1.15,-11,;-.51,-9.59,)| Show InChI InChI=1S/C38H36N2O6/c1-17-13-21-23-15-25(43-7)33(41)27(31(23)39-29(21)19-9-11-37(3,4)45-35(17)19)28-32-24(16-26(44-8)34(28)42)22-14-18(2)36-20(30(22)40-32)10-12-38(5,6)46-36/h9-16,39-42H,1-8H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin K |
Bioorg Med Chem Lett 16: 6178-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.042
BindingDB Entry DOI: 10.7270/Q2C25069 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50323198

(4'''-methylamentoflavone | CHEMBL220745 | podocarp...)Show SMILES COc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(9.76,-25.42,;8.22,-25.42,;7.45,-24.09,;8.22,-22.76,;7.45,-21.42,;5.91,-21.42,;5.14,-22.76,;5.91,-24.09,;5.14,-20.09,;5.91,-18.76,;5.14,-17.43,;5.91,-16.09,;3.6,-17.43,;2.83,-16.09,;3.6,-14.76,;1.29,-16.09,;.52,-17.43,;-1.02,-17.43,;1.29,-18.76,;.52,-20.09,;-1.02,-20.09,;-1.79,-21.42,;-1.02,-22.76,;.52,-22.76,;1.29,-21.42,;2.83,-21.42,;-3.33,-21.42,;-4.1,-20.09,;-5.64,-20.09,;-6.41,-18.76,;-6.41,-21.42,;-5.64,-22.76,;-6.41,-24.09,;-7.95,-24.09,;-8.72,-25.42,;-8.72,-22.76,;-7.95,-21.42,;-8.72,-20.09,;-4.1,-22.76,;2.83,-18.76,;3.6,-20.09,)| Show InChI InChI=1S/C31H20O10/c1-39-17-5-2-14(3-6-17)25-13-24(38)30-22(36)11-21(35)28(31(30)41-25)18-8-15(4-7-19(18)33)26-12-23(37)29-20(34)9-16(32)10-27(29)40-26/h2-13,32-33,35-37H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin K |
Bioorg Med Chem Lett 16: 6178-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.042
BindingDB Entry DOI: 10.7270/Q2C25069 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50476092

(Aniasatin)Show SMILES [H][C@@]12C[C@]3([C@H](C)C[C@@H](O)[C@]3(O)[C@]3(COC3=O)[C@@]1(C)O)[C@@H](O)C(=O)O2 Show InChI InChI=1S/C15H20O8/c1-6-3-7(16)15(21)13(6)4-8(23-10(18)9(13)17)12(2,20)14(15)5-22-11(14)19/h6-9,16-17,20-21H,3-5H2,1-2H3/t6-,7-,8-,9+,12+,13+,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 by quenched fluorescense assay |
Bioorg Med Chem Lett 16: 6178-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.042
BindingDB Entry DOI: 10.7270/Q2C25069 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50476090
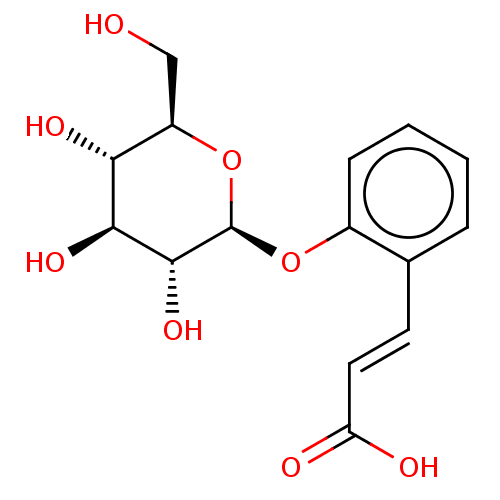
(CHEBI:17531 | MELILOTOSIDE)Show SMILES OC[C@H]1O[C@@H](Oc2ccccc2\C=C\C(O)=O)[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C15H18O8/c16-7-10-12(19)13(20)14(21)15(23-10)22-9-4-2-1-3-8(9)5-6-11(17)18/h1-6,10,12-16,19-21H,7H2,(H,17,18)/b6-5+/t10-,12-,13+,14-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-v-beta-3 integrin receptor by ELISA |
Bioorg Med Chem Lett 16: 6178-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.042
BindingDB Entry DOI: 10.7270/Q2C25069 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131046

(8,13-Dihydro-7H-indolo[2',3':3,4]pyrido[2,1-b]quin...)Show InChI InChI=1S/C18H13N3O/c22-18-13-6-2-4-8-15(13)20-17-16-12(9-10-21(17)18)11-5-1-3-7-14(11)19-16/h1-8,19H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 by quenched fluorescense assay |
Bioorg Med Chem Lett 16: 6178-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.042
BindingDB Entry DOI: 10.7270/Q2C25069 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data