

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50438479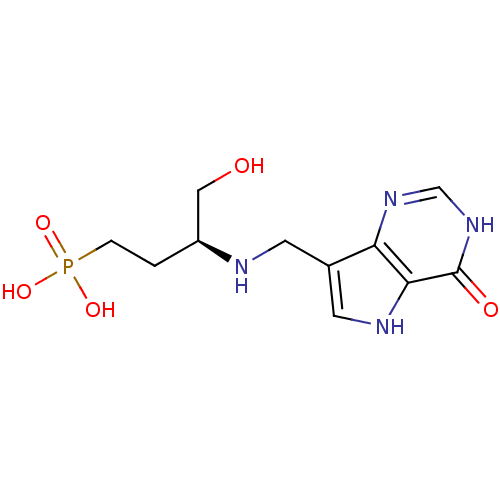 (CHEMBL2414636) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 385 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human HGPRT by Morrison method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02184 BindingDB Entry DOI: 10.7270/Q26D5XRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxanthine-guanine phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50438487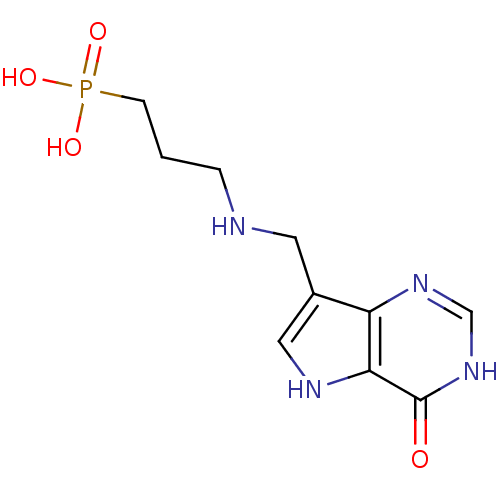 (CHEMBL2414703) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human HGPRT by Morrison method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02184 BindingDB Entry DOI: 10.7270/Q26D5XRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50501284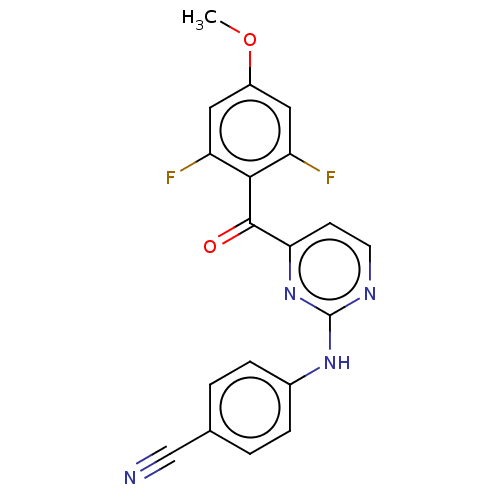 (CHEMBL3944697) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 347 | n/a | n/a | n/a | n/a |
Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV-1 3B reverse transcriptase K103N mutant infected in human MT4 cells assessed as inhibition of viral replication after 5 days by cel... | Eur J Med Chem 122: 185-195 (2016) Article DOI: 10.1016/j.ejmech.2016.06.026 BindingDB Entry DOI: 10.7270/Q2NC647C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50501284 (CHEMBL3944697) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | >500 | n/a | n/a | n/a | n/a |
Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of reverse transcriptase Y181C mutant in HIV-1 3B infected in human MT4 cells assessed as inhibition of viral replication after 5 days by ... | Eur J Med Chem 122: 185-195 (2016) Article DOI: 10.1016/j.ejmech.2016.06.026 BindingDB Entry DOI: 10.7270/Q2NC647C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of reverse transcriptase Y181C mutant in HIV-1 3B infected in human MT4 cells assessed as inhibition of viral replication after 5 days by ... | Eur J Med Chem 122: 185-195 (2016) Article DOI: 10.1016/j.ejmech.2016.06.026 BindingDB Entry DOI: 10.7270/Q2NC647C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV-1 3B reverse transcriptase K103N mutant infected in human MT4 cells assessed as inhibition of viral replication after 5 days by cel... | Eur J Med Chem 122: 185-195 (2016) Article DOI: 10.1016/j.ejmech.2016.06.026 BindingDB Entry DOI: 10.7270/Q2NC647C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of reverse transcriptase Y181C mutant in HIV-1 3B infected in human MT4 cells assessed as inhibition of viral replication after 5 days by ... | Eur J Med Chem 122: 185-195 (2016) Article DOI: 10.1016/j.ejmech.2016.06.026 BindingDB Entry DOI: 10.7270/Q2NC647C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 101 | n/a | n/a | n/a | n/a |
Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV-1 3B reverse transcriptase K103N mutant infected in human MT4 cells assessed as inhibition of viral replication after 5 days by cel... | Eur J Med Chem 122: 185-195 (2016) Article DOI: 10.1016/j.ejmech.2016.06.026 BindingDB Entry DOI: 10.7270/Q2NC647C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||