 Found 398 hits with Last Name = 'di fruscia' and Initial = 'p'
Found 398 hits with Last Name = 'di fruscia' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50148827
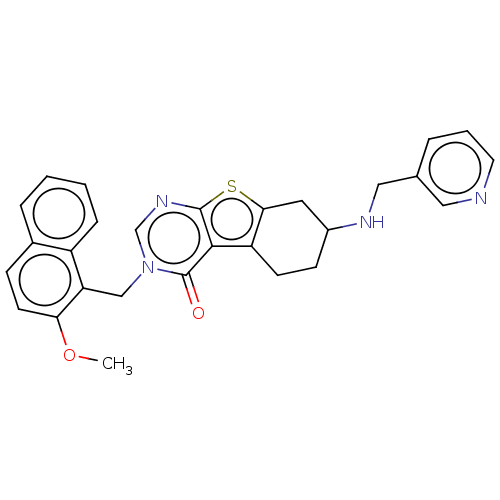
(CHEMBL3769975)Show SMILES COc1ccc2ccccc2c1Cn1cnc2sc3CC(CCc3c2c1=O)NCc1cccnc1 Show InChI InChI=1S/C28H26N4O2S/c1-34-24-11-8-19-6-2-3-7-21(19)23(24)16-32-17-31-27-26(28(32)33)22-10-9-20(13-25(22)35-27)30-15-18-5-4-12-29-14-18/h2-8,11-12,14,17,20,30H,9-10,13,15-16H2,1H3 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli using tubulin-K40 peptide in prese... |
J Med Chem 60: 1928-1945 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01690
BindingDB Entry DOI: 10.7270/Q23F4SP1 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM553940
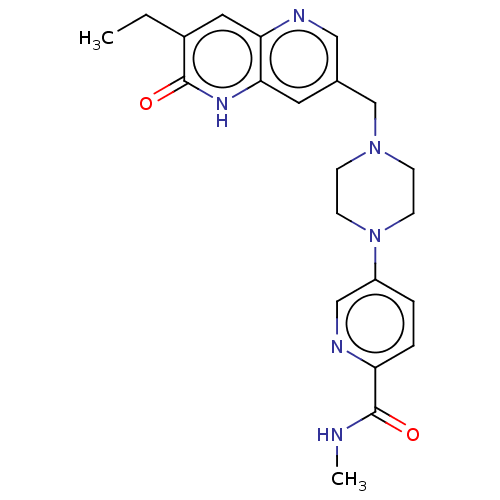
(US11325906, Example 4)Show SMILES CCc1cc2ncc(CN3CCN(CC3)c3ccc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM553937
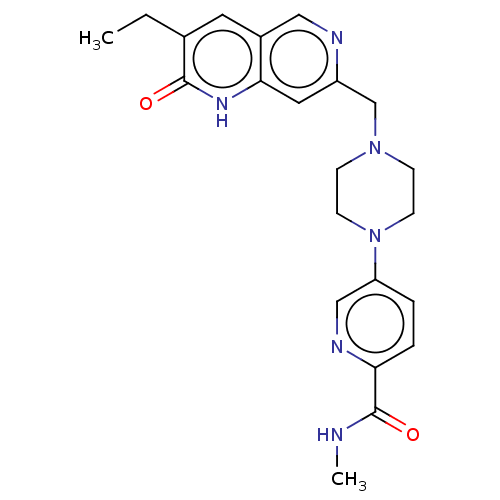
(US11325906, Example 1)Show SMILES CCc1cc2cnc(CN3CCN(CC3)c3ccc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM85050
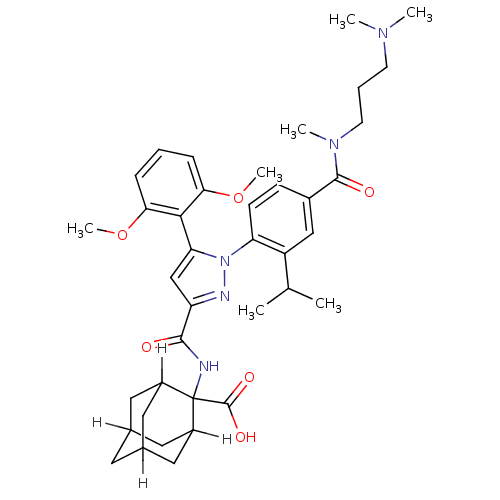
(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at NTSR1 (unknown origin) expressed in CHO cells assessed as inhibition of NT(8-13) peptide-induced change in intracellular Ca2+ ... |
Bioorg Med Chem Lett 24: 3974-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.033
BindingDB Entry DOI: 10.7270/Q2ZP47RG |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601780
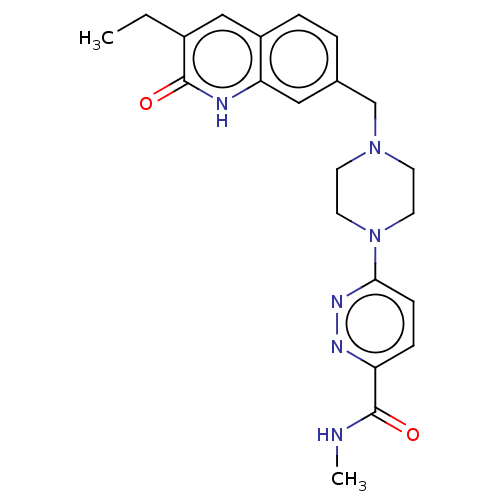
(CHEMBL5202060)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3ccc(nn3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601781
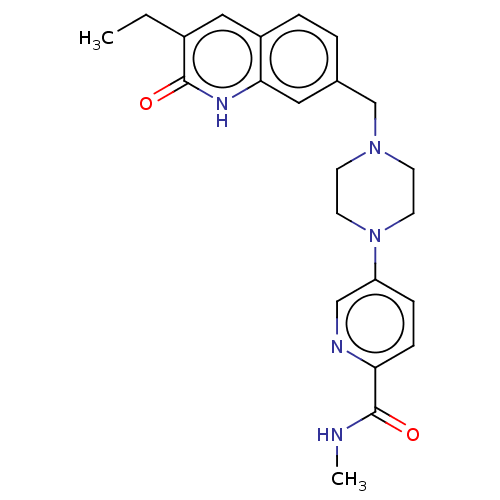
(CHEMBL5197101)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3ccc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM553947
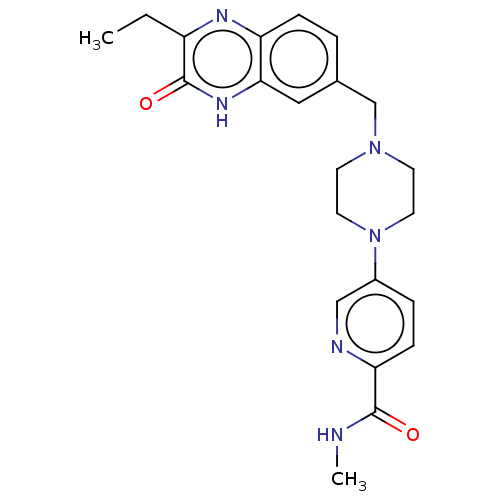
(US11325906, Example 11)Show SMILES CCc1nc2ccc(CN3CCN(CC3)c3ccc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601767
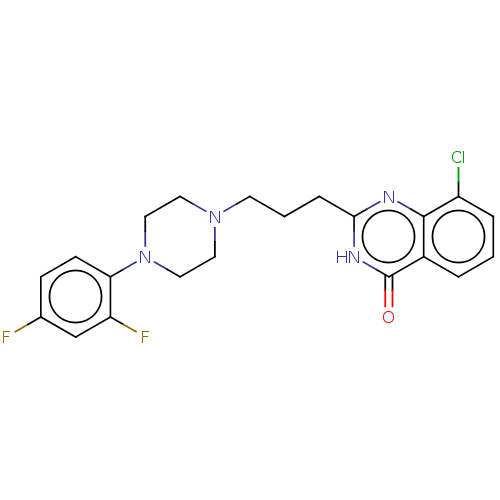
(CHEMBL5190481)Show SMILES Fc1ccc(N2CCN(CCCc3nc4c(Cl)cccc4c(=O)[nH]3)CC2)c(F)c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601779
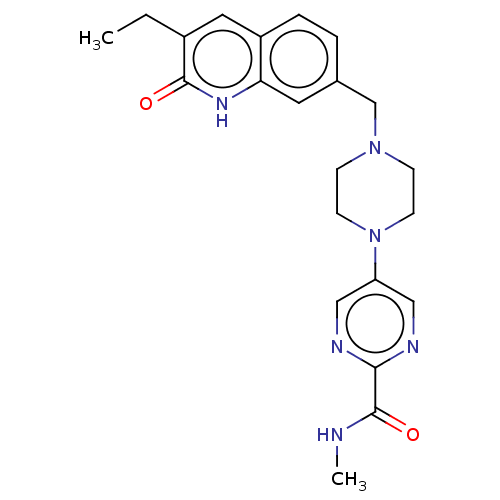
(CHEMBL5183769)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3cnc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601776
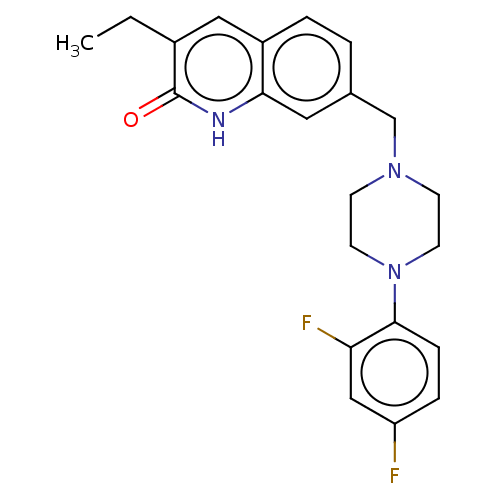
(CHEMBL5196187)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3ccc(F)cc3F)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601772
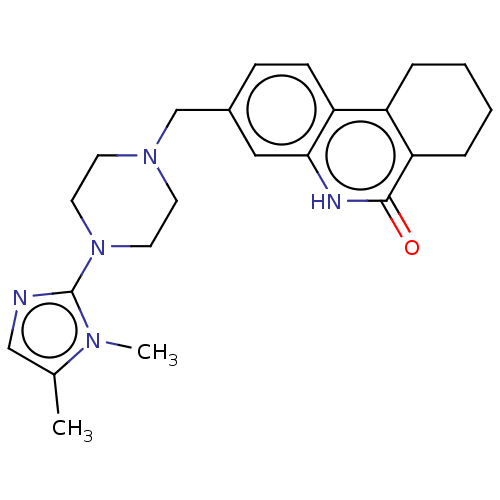
(CHEMBL5177596)Show SMILES Cc1cnc(N2CCN(Cc3ccc4c5CCCCc5c(=O)[nH]c4c3)CC2)n1C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50084621
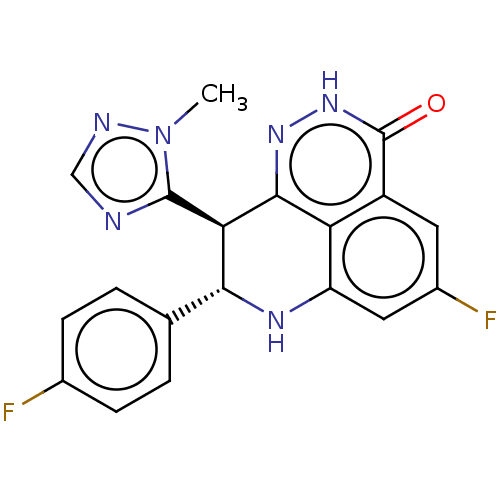
(BMN 673 | Talazoparib)Show SMILES Cn1ncnc1[C@@H]1[C@H](Nc2cc(F)cc3c2c1n[nH]c3=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H14F2N6O/c1-27-18(22-8-23-27)15-16(9-2-4-10(20)5-3-9)24-13-7-11(21)6-12-14(13)17(15)25-26-19(12)28/h2-8,15-16,24H,1H3,(H,26,28)/t15-,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601769
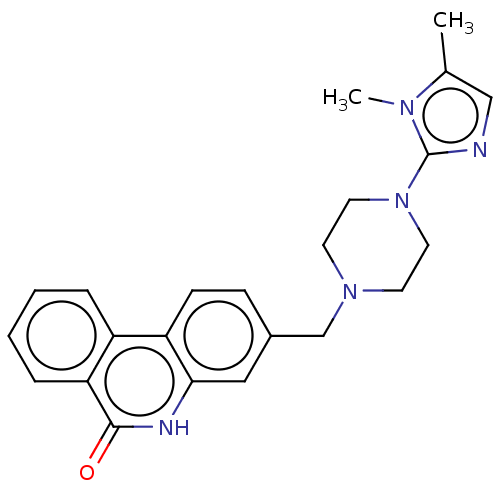
(CHEMBL5191396)Show SMILES Cc1cnc(N2CCN(Cc3ccc4c(c3)[nH]c(=O)c3ccccc43)CC2)n1C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601768
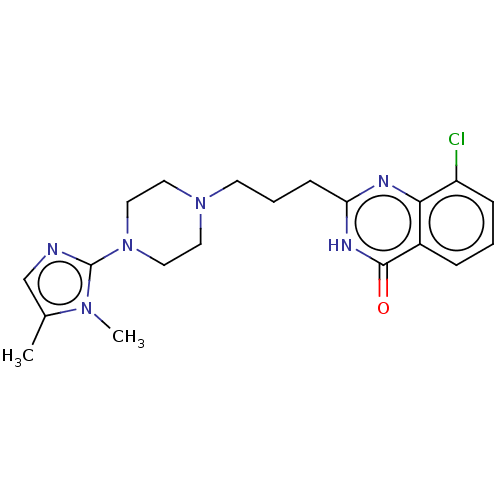
(CHEMBL5193246)Show SMILES Cc1cnc(N2CCN(CCCc3nc4c(Cl)cccc4c(=O)[nH]3)CC2)n1C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM209932
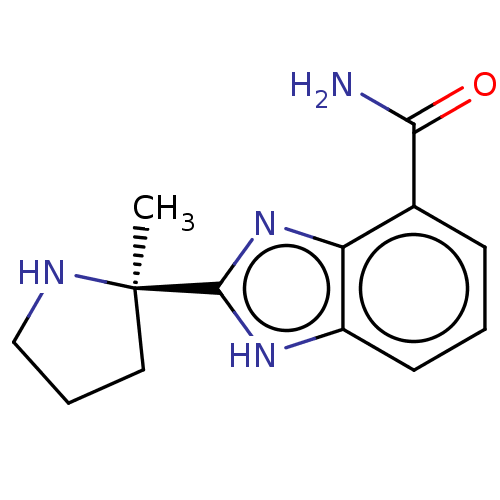
(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577403
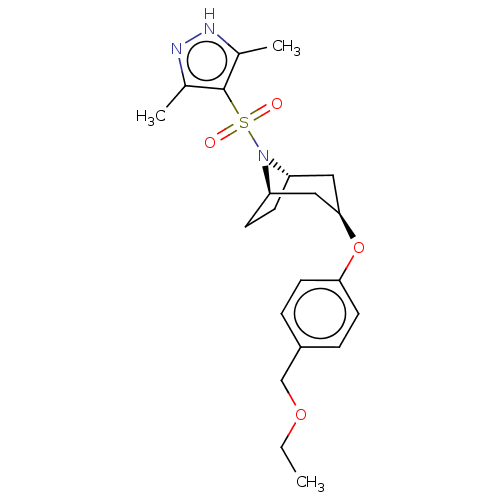
(CHEMBL4878464)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1ccc(COCC)cc1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:20:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577404
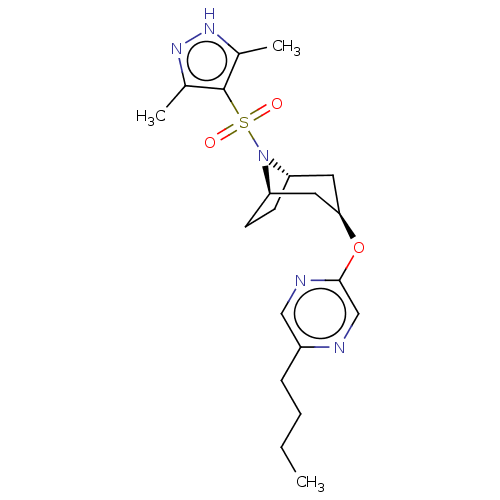
(CHEMBL4864438)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1cnc(CCCC)cn1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:20:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601777
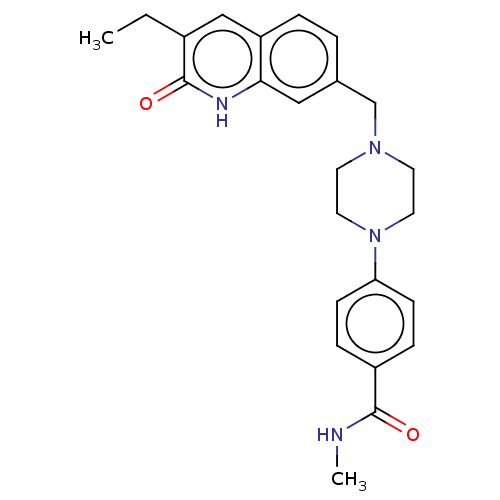
(CHEMBL5200723)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3ccc(cc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577396

(CHEMBL4863119)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1ccc(CCCCCC)cc1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:22:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577394
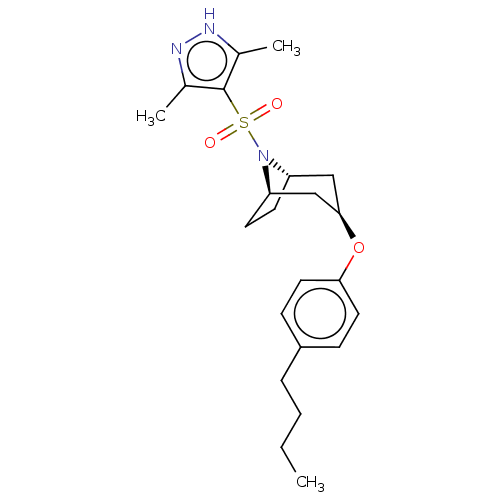
(CHEMBL4848541)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1ccc(CCCC)cc1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:20:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601775

(CHEMBL5181905)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3ncc(C)n3C)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601778

(CHEMBL5195580)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3cnc(cn3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50084621

(BMN 673 | Talazoparib)Show SMILES Cn1ncnc1[C@@H]1[C@H](Nc2cc(F)cc3c2c1n[nH]c3=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H14F2N6O/c1-27-18(22-8-23-27)15-16(9-2-4-10(20)5-3-9)24-13-7-11(21)6-12-14(13)17(15)25-26-19(12)28/h2-8,15-16,24H,1H3,(H,26,28)/t15-,16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601770

(CHEMBL5197824)Show SMILES Cc1cnc(N2CCN(Cc3ccc4c(c3)[nH]c(=O)c3cccnc43)CC2)n1C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50316226
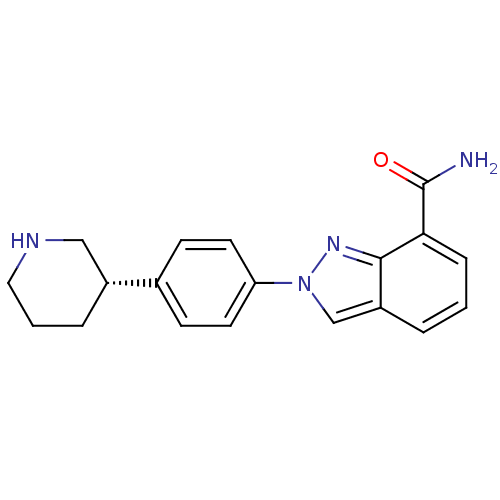
((S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...)Show SMILES NC(=O)c1cccc2cn(nc12)-c1ccc(cc1)[C@@H]1CCCNC1 |r| Show InChI InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577388
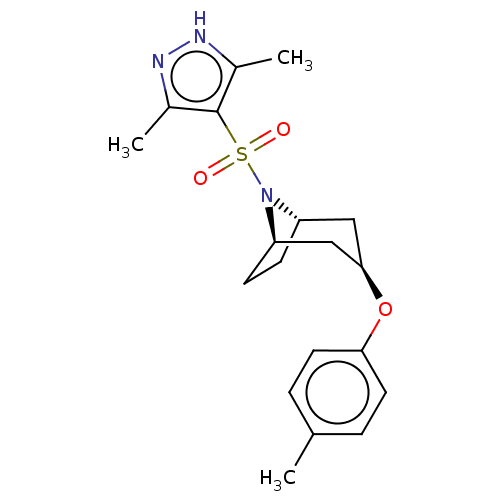
(CHEMBL4871884)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1ccc(C)cc1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:17:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601773

(CHEMBL5178594)Show SMILES Cc1cnc(N2CCN(Cc3ccc4c5CCOCc5c(=O)[nH]c4c3)CC2)n1C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577405
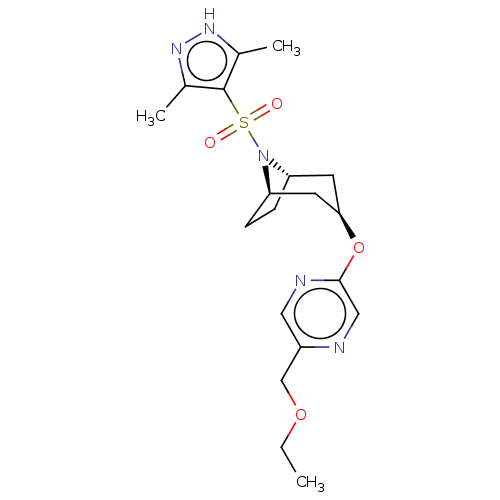
(CHEMBL4875895)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1cnc(COCC)cn1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:20:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601774
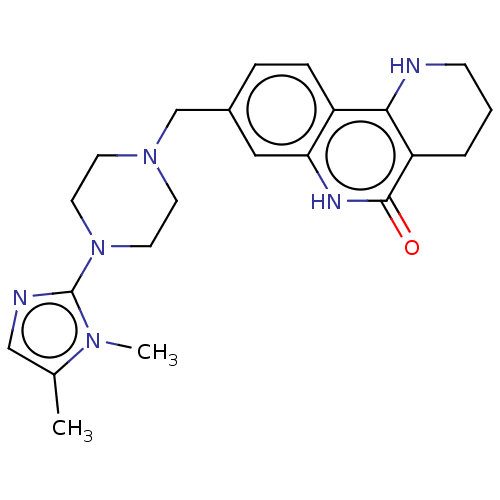
(CHEMBL5209181)Show SMILES Cc1cnc(N2CCN(Cc3ccc4c5NCCCc5c(=O)[nH]c4c3)CC2)n1C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577392
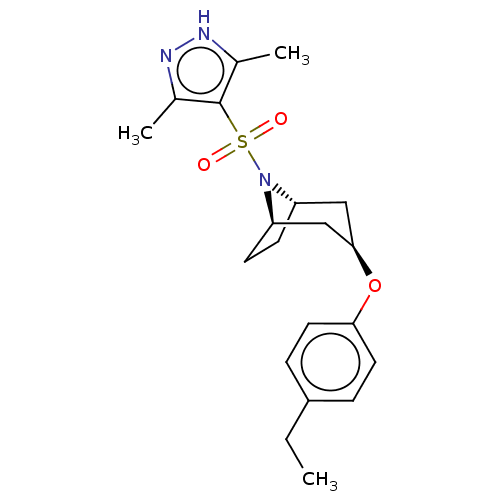
(CHEMBL4875599)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1ccc(CC)cc1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:18:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27708
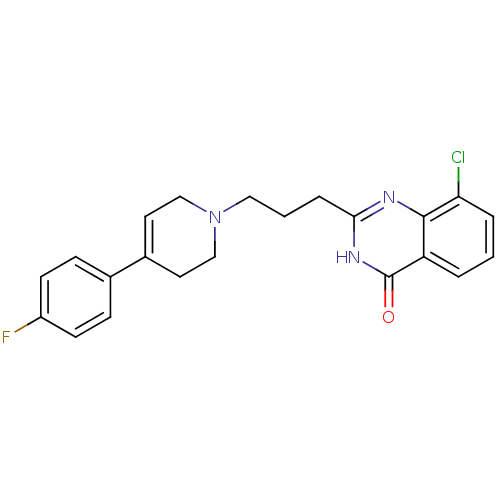
(8-chloro-2-{3-[4-(4-fluorophenyl)-1,2,3,6-tetrahyd...)Show SMILES Fc1ccc(cc1)C1=CCN(CCCc2nc3c(Cl)cccc3c(=O)[nH]2)CC1 |t:8| Show InChI InChI=1S/C22H21ClFN3O/c23-19-4-1-3-18-21(19)25-20(26-22(18)28)5-2-12-27-13-10-16(11-14-27)15-6-8-17(24)9-7-15/h1,3-4,6-10H,2,5,11-14H2,(H,25,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM138348
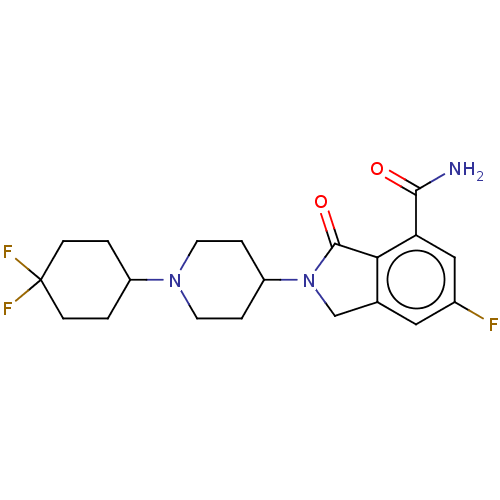
(US8877944, 99)Show SMILES NC(=O)c1cc(F)cc2CN(C3CCN(CC3)C3CCC(F)(F)CC3)C(=O)c12 Show InChI InChI=1S/C20H24F3N3O2/c21-13-9-12-11-26(19(28)17(12)16(10-13)18(24)27)15-3-7-25(8-4-15)14-1-5-20(22,23)6-2-14/h9-10,14-15H,1-8,11H2,(H2,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601771
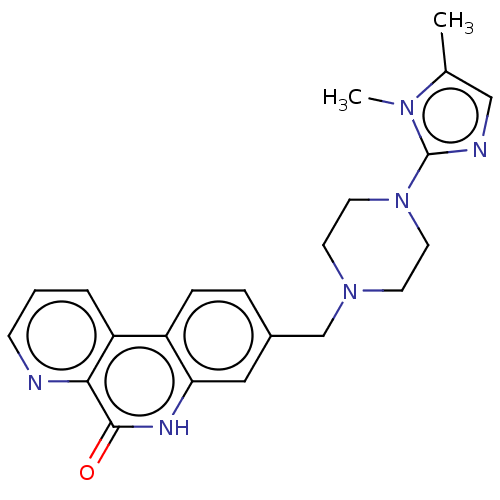
(CHEMBL5176615)Show SMILES Cc1cnc(N2CCN(Cc3ccc4c(c3)[nH]c(=O)c3ncccc43)CC2)n1C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM50248034
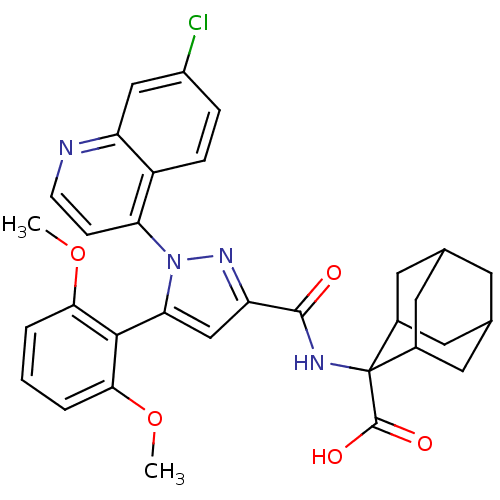
(2-{[1-(7-Chloro-quinolin-4-yl)-5-(2,6-dimethoxy-ph...)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccnc2cc(Cl)ccc12)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:38:37:35:31.32.33,39:29:35:31.32.33,28:29:31.38.32:36.34.35,THB:38:32:29.37.36:35,39:29:31.38.32:36.34.35,28:29:35:31.32.33,33:32:29:36.34.35,33:34:29:31.38.32,(-7.63,.4,;-6.3,-.36,;-6.29,-1.9,;-7.62,-2.68,;-7.62,-4.22,;-6.29,-4.99,;-4.95,-4.22,;-3.61,-4.99,;-3.61,-6.53,;-4.95,-2.67,;-3.63,-1.9,;-2.22,-2.52,;-1.19,-1.37,;-1.97,-.04,;-3.47,-.36,;-4.62,.66,;-6.08,.17,;-7.22,1.19,;-6.91,2.71,;-5.45,3.19,;-5.14,4.69,;-3.68,5.16,;-3.37,6.67,;-2.53,4.14,;-2.85,2.64,;-4.31,2.16,;.35,-1.53,;.98,-2.93,;1.24,-.27,;2.78,-.43,;3.43,1.2,;4.84,1.23,;6.02,2.07,;5.46,3.5,;3.95,3.52,;2.87,2.58,;3.44,1.99,;4.02,.52,;5.54,.51,;3.42,-1.84,;2.51,-3.09,;4.95,-2,)| Show InChI InChI=1S/C32H31ClN4O5/c1-41-27-4-3-5-28(42-2)29(27)26-16-24(36-37(26)25-8-9-34-23-15-21(33)6-7-22(23)25)30(38)35-32(31(39)40)19-11-17-10-18(13-19)14-20(32)12-17/h3-9,15-20H,10-14H2,1-2H3,(H,35,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at NTSR1 (unknown origin) expressed in CHO cells assessed as inhibition of NT(8-13) peptide-induced change in intracellular Ca2+ ... |
Bioorg Med Chem Lett 24: 3974-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.033
BindingDB Entry DOI: 10.7270/Q2ZP47RG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577386
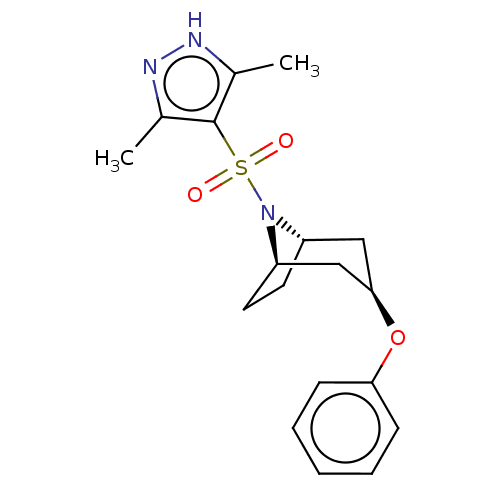
(CHEMBL4861341)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1ccccc1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:16:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577397

(CHEMBL4877336)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1ccc(CCC(C)C)cc1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:21:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577401
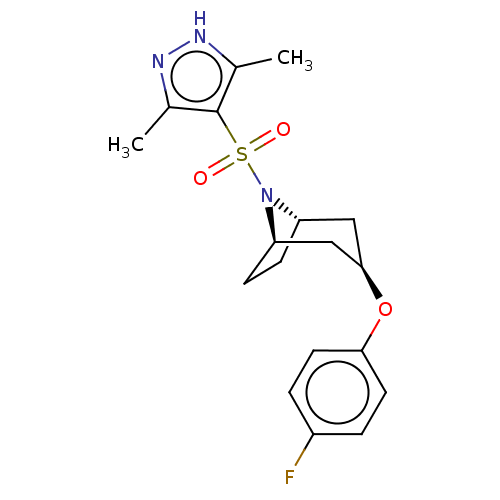
(CHEMBL4873115)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1ccc(F)cc1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:17:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577385
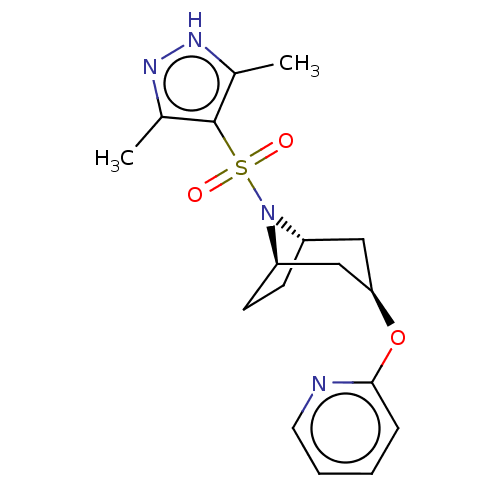
(CHEMBL4861841)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1ccccn1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:16:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50148827

(CHEMBL3769975)Show SMILES COc1ccc2ccccc2c1Cn1cnc2sc3CC(CCc3c2c1=O)NCc1cccnc1 Show InChI InChI=1S/C28H26N4O2S/c1-34-24-11-8-19-6-2-3-7-21(19)23(24)16-32-17-31-27-26(28(32)33)22-10-9-20(13-25(22)35-27)30-15-18-5-4-12-29-14-18/h2-8,11-12,14,17,20,30H,9-10,13,15-16H2,1H3 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged SIRT2 (50 to 389 end residues) expressed in Escherichia coli in presence of NAD+ by enzyme coup... |
J Med Chem 60: 1928-1945 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01690
BindingDB Entry DOI: 10.7270/Q23F4SP1 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577399

(CHEMBL4879033)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1ccc(OC)cc1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:18:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577398
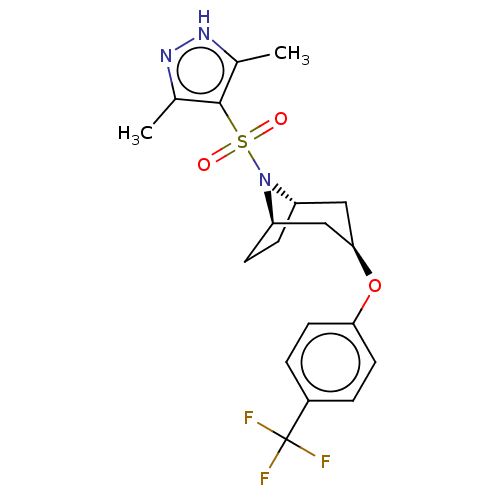
(CHEMBL4857016)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1ccc(cc1)C(F)(F)F)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:20:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM209932

(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577380
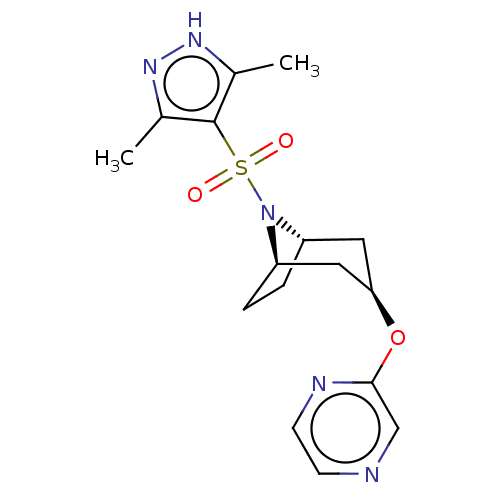
(CHEMBL4848387)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1cnccn1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:16:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577391
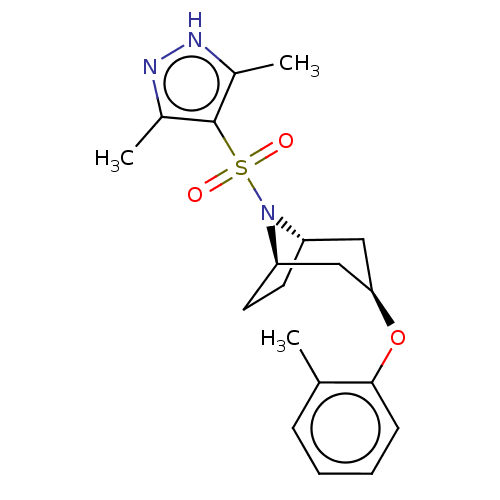
(CHEMBL4869873)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Oc1ccccc1C)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:17:2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577376

(CHEMBL4866583)Show SMILES CCCc1n[nH]c(C)c1S(=O)(=O)N1CCC(CC1)Oc1cnccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50577381
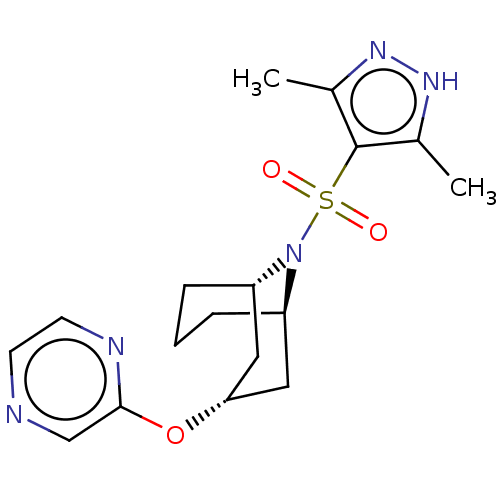
(CHEMBL4865423)Show SMILES [H][C@]12CCC[C@]([H])(C[C@@H](C1)Oc1cnccn1)N2S(=O)(=O)c1c(C)n[nH]c1C |r,TLB:18:17:2.3.4:7.8.9,10:8:2.3.4:17| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK293 cells using PAMCA as fluorogenic substrate preincubated for 30 mins followed by substrate ad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00575
BindingDB Entry DOI: 10.7270/Q2P272ZJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data