

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50269061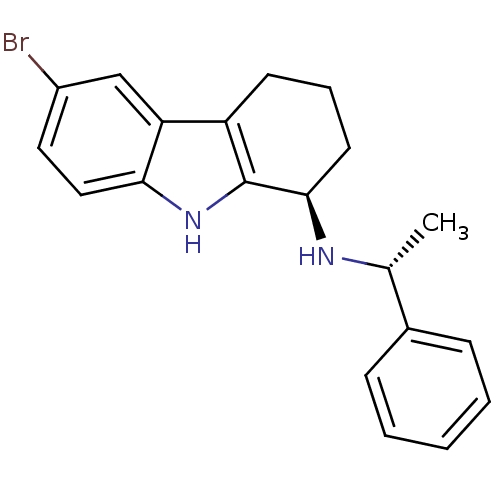 ((1R)-6-Bromo-N-[(1R)-1-phenylethyl]-2,3,4,9-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50269061 ((1R)-6-Bromo-N-[(1R)-1-phenylethyl]-2,3,4,9-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50295256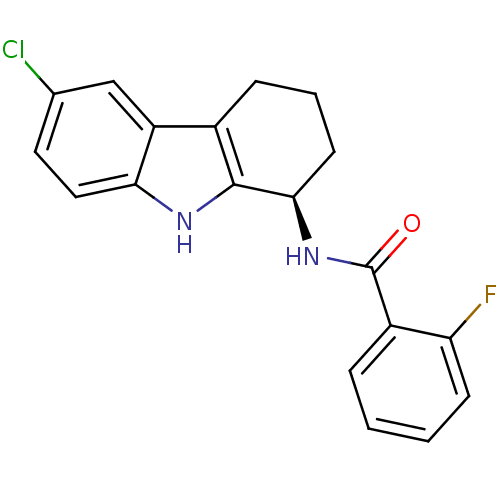 ((R)-N-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50295257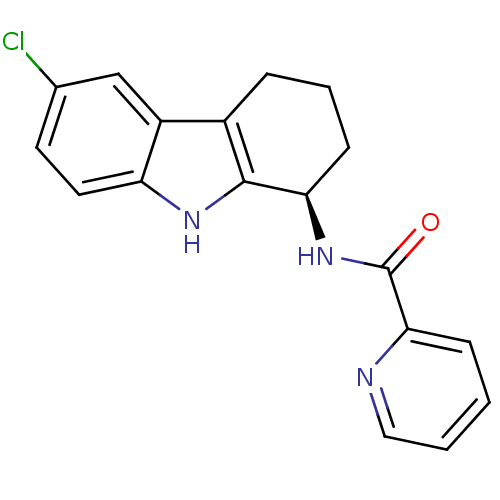 ((R)-N-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50295256 ((R)-N-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50295257 ((R)-N-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50295255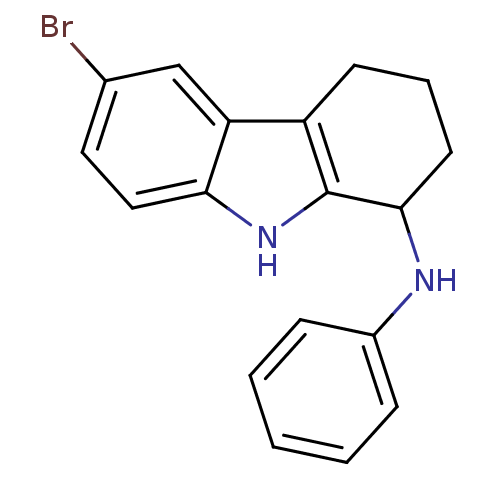 (6-bromo-N-phenyl-2,3,4,9-tetrahydro-1H-carbazol-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50295255 (6-bromo-N-phenyl-2,3,4,9-tetrahydro-1H-carbazol-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 19: 4110-4 (2009) Article DOI: 10.1016/j.bmcl.2009.06.001 BindingDB Entry DOI: 10.7270/Q27944RX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263072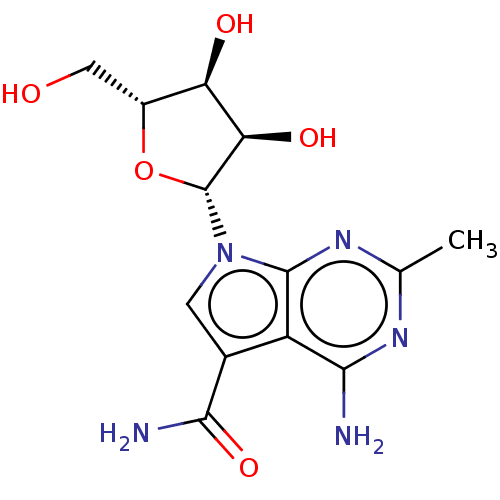 (US9708359, 1 | US9708359, 122 | US9708359, 38 | US...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263073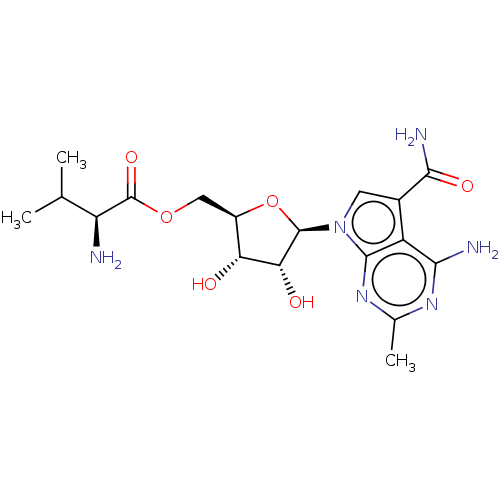 (US9708359, 2) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263074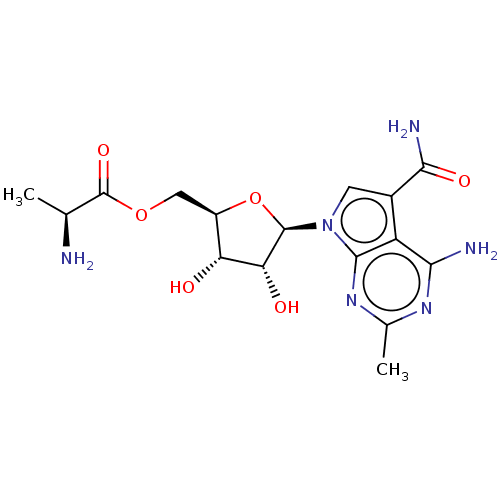 (US9708359, 3) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263075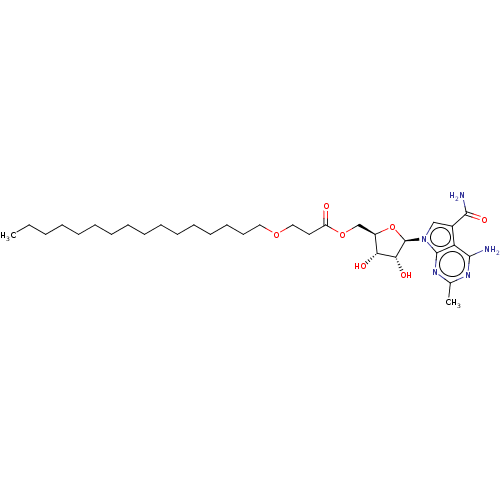 (US9708359, 4) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263076 (US9708359, 5) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263072 (US9708359, 1 | US9708359, 122 | US9708359, 38 | US...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.08E+3 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263078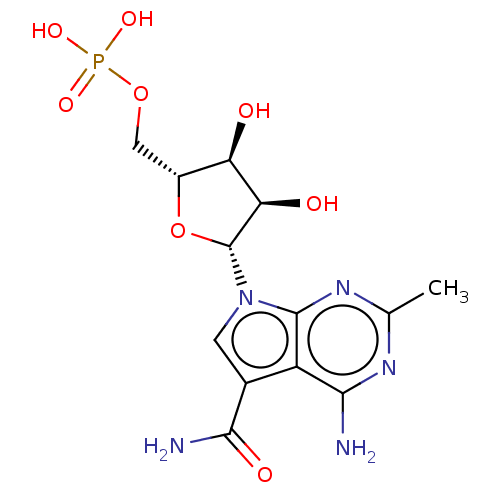 (US9708359, 71) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.15E+3 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263079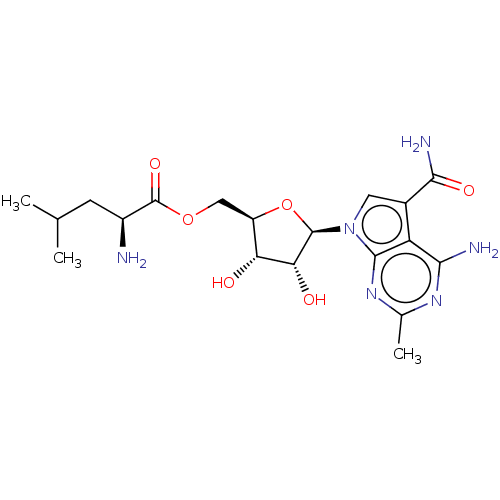 (US9708359, 77) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263080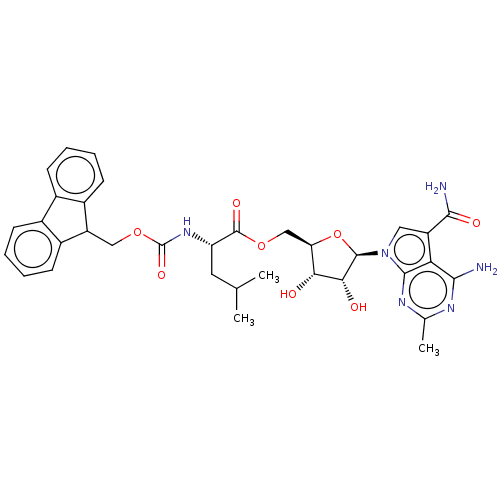 (US9708359, 76) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263081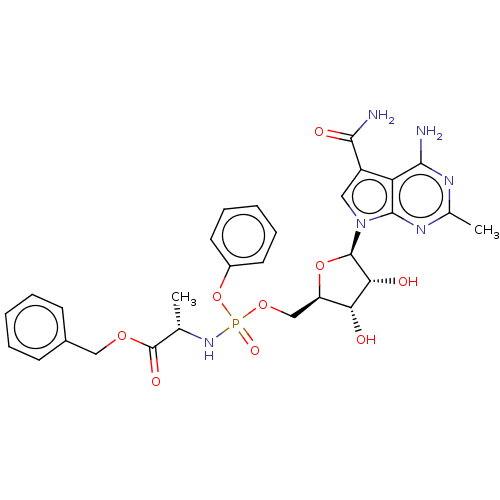 (US9708359, 107) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263082 (US9708359, 111) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263083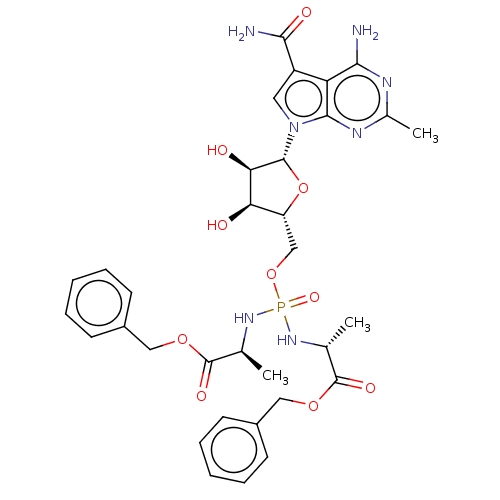 (US9708359, 126) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.36E+4 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263083 (US9708359, 126) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.89E+4 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263084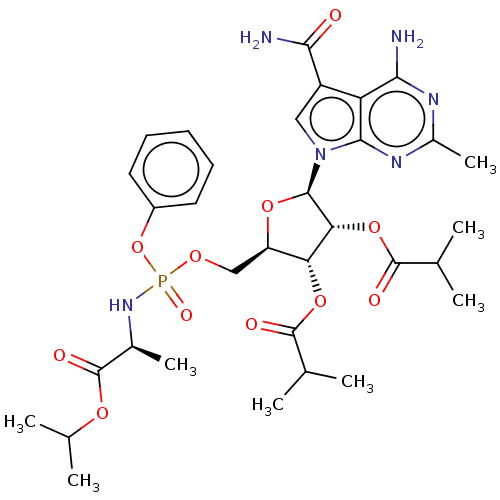 (US9708359, 133) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263085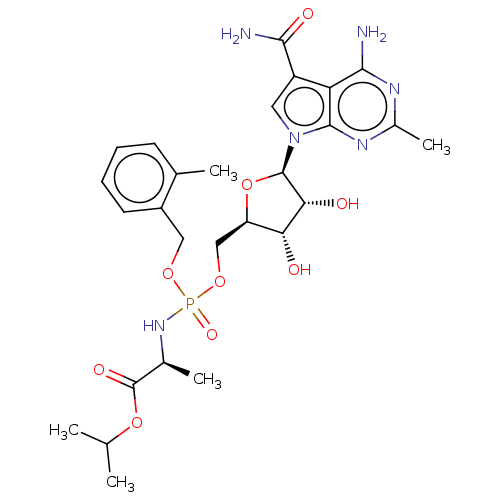 (US9708359, 137) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263087 (US9708359, 141) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263088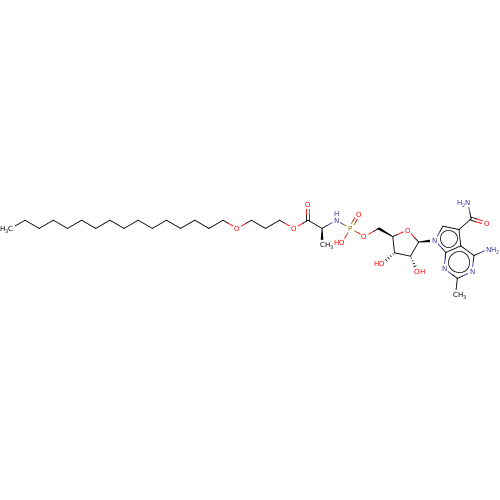 (US9708359, 143) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263089 (US9708359, 145) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263090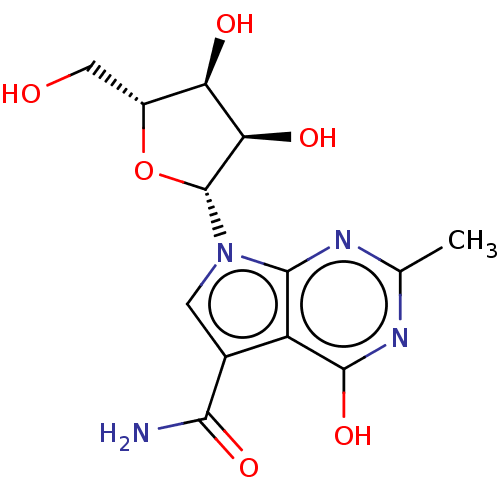 (US9708359, 7) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 5.38E+4 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263091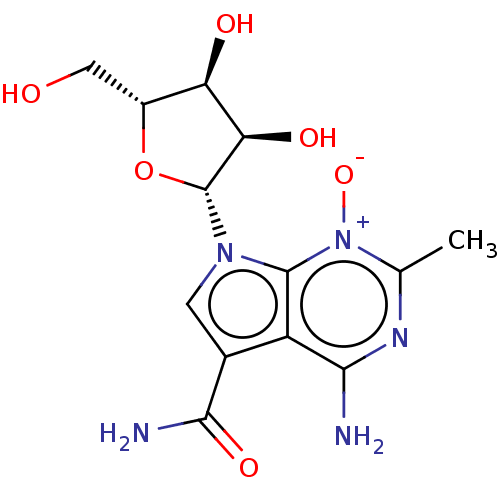 (US9708359, 8) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263092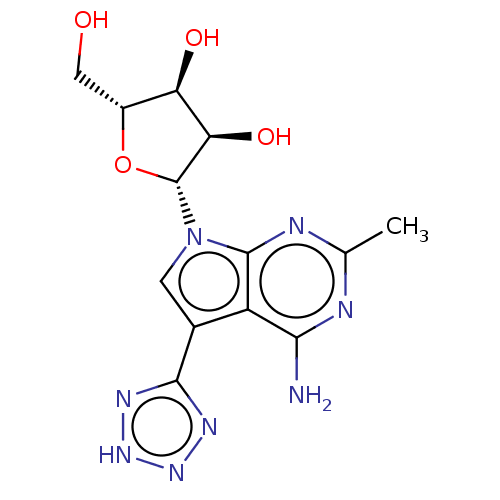 (US9708359, 10) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.15E+4 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263094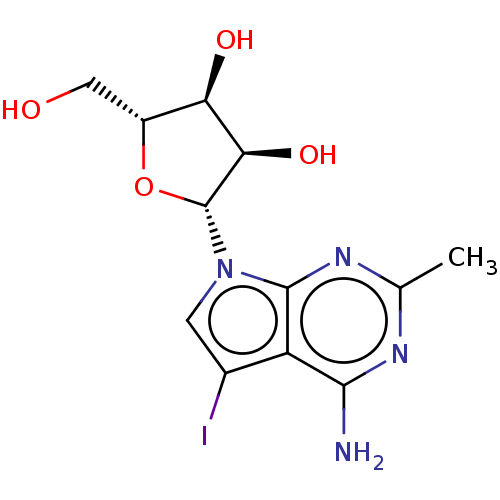 (US9708359, 14) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263095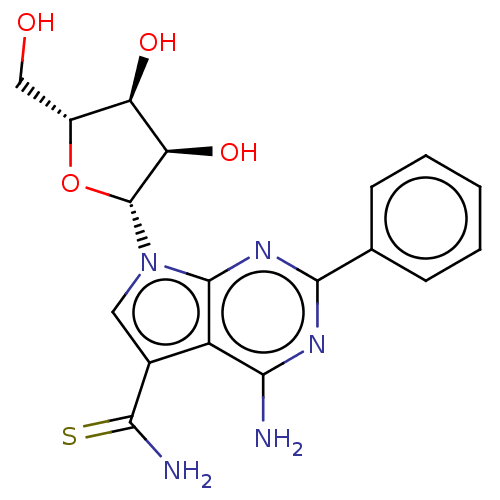 (US9708359, 16 | US9708359, 28) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263096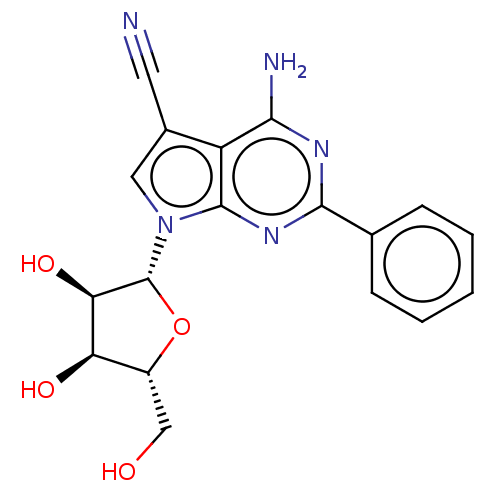 (US9708359, 17) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263097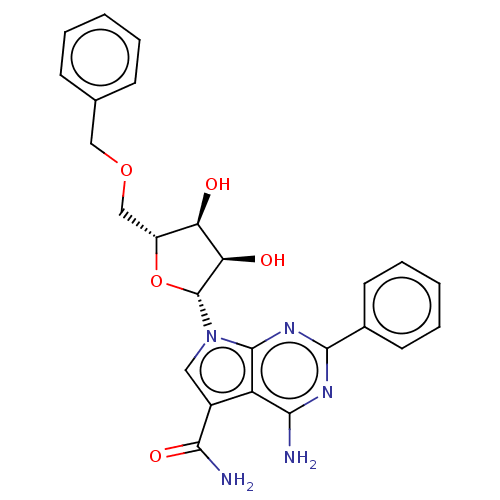 (US9708359, 18) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263098 (US9708359, 19) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263099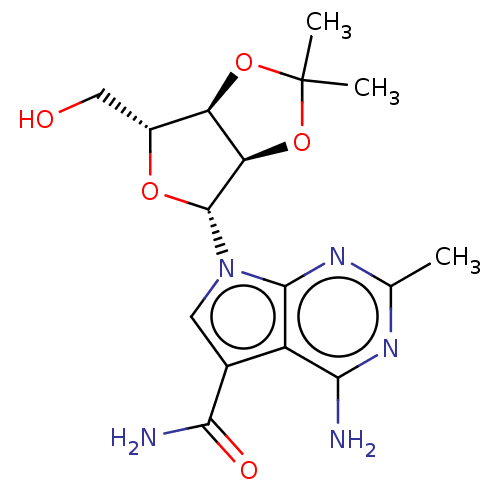 (US9708359, 20) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263101 (US9708359, 22) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263102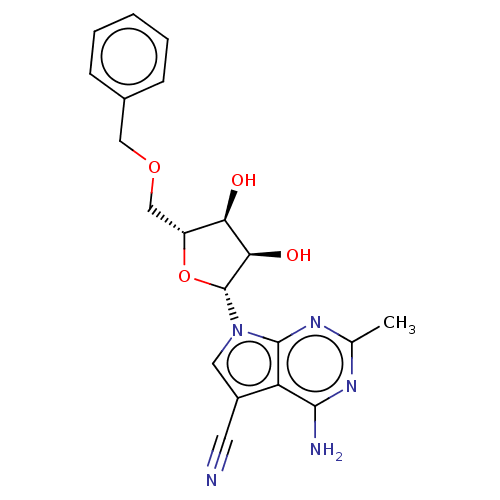 (US9708359, 27) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263095 (US9708359, 16 | US9708359, 28) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263104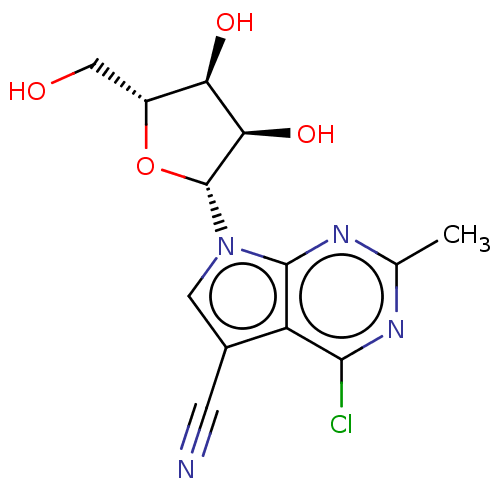 (US9708359, 29) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263105 (US9708359, 30) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263107 (US9708359, 32) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263108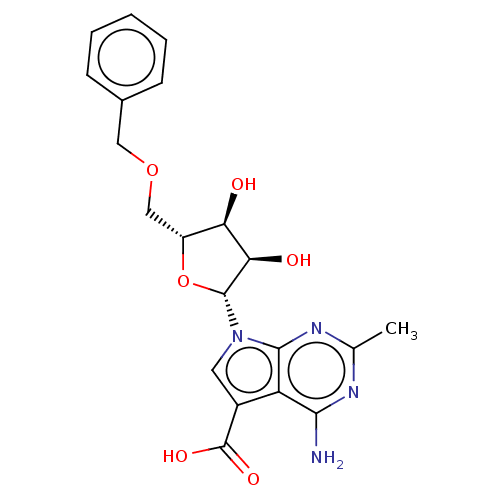 (US9708359, 33) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263109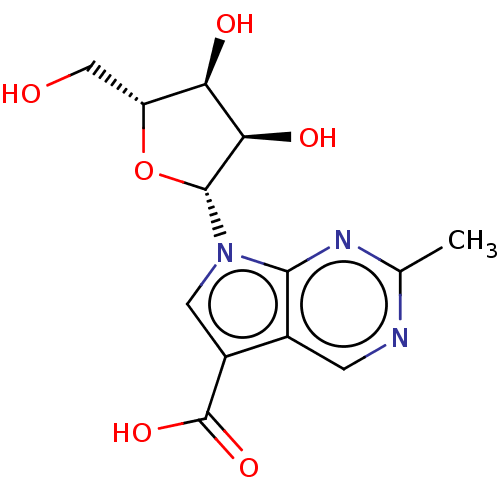 (US9708359, 34) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263110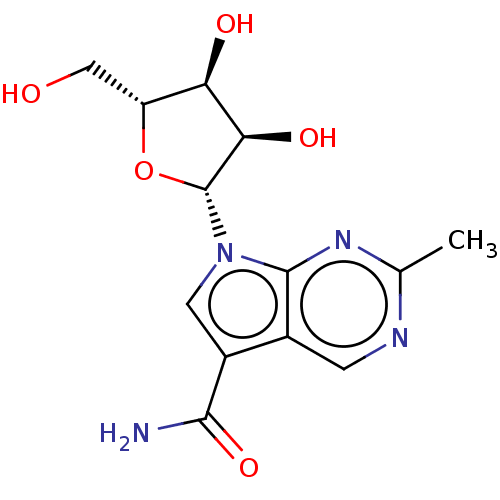 (US9708359, 35) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263111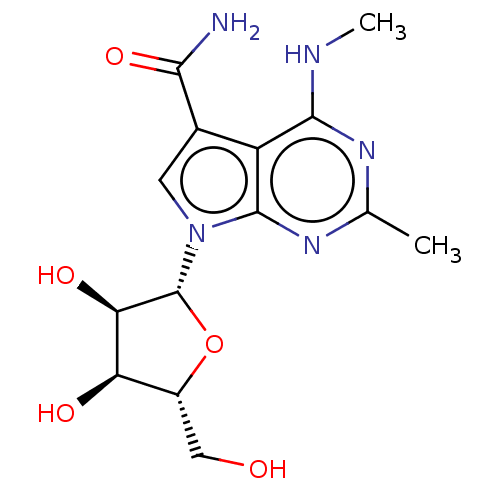 (US9708359, 36) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263112 (US9708359, 37) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263114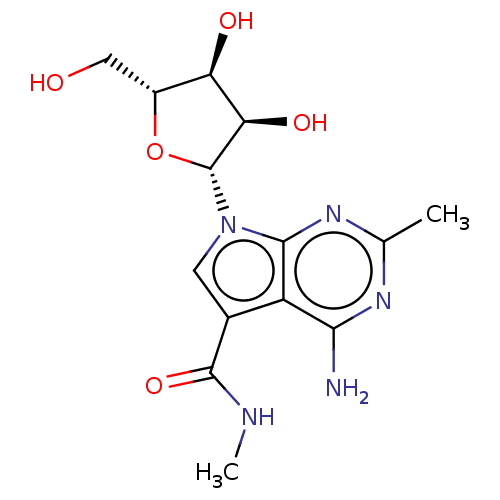 (US9708359, 39) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263115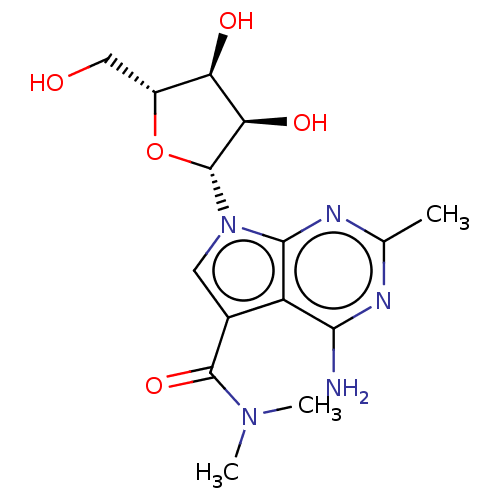 (US9708359, 41) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263116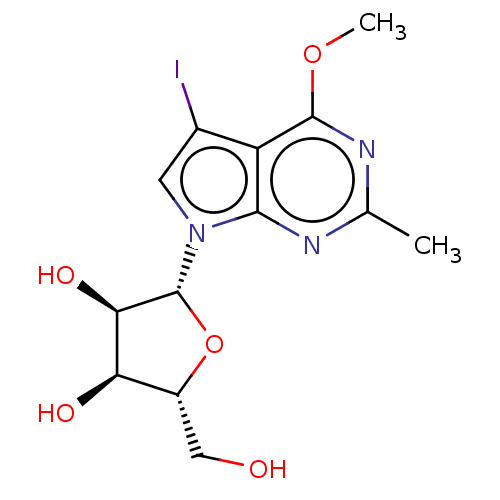 (US9708359, 42) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Murine norovirus 1) | BDBM263117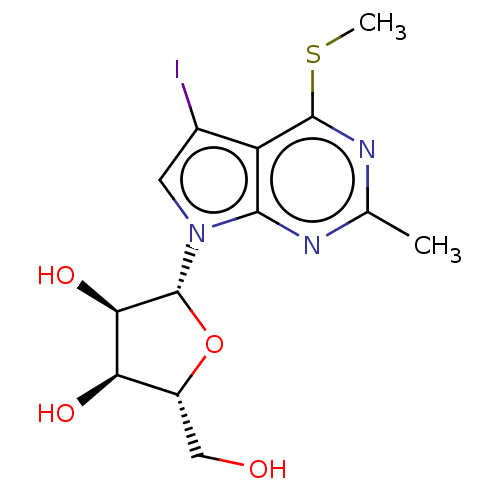 (US9708359, 43) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Chimerix, Inc.; The Regents of the University of Michigan US Patent | Assay Description Polymerase reactions (10 μL) were conducted for 60 minutes at 37° C. Nucleoside triphosphates (NTPs) were present at 100 μM each, with 0.05... | US Patent US9708359 (2017) BindingDB Entry DOI: 10.7270/Q22F7QGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 151 total ) | Next | Last >> |