

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217448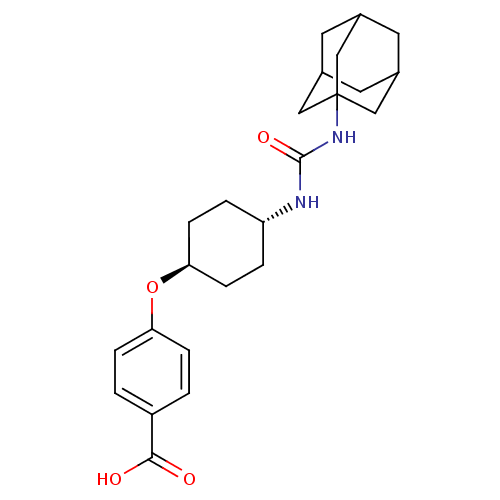 (CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587459 (CHEMBL5092295) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM25737 (12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM81356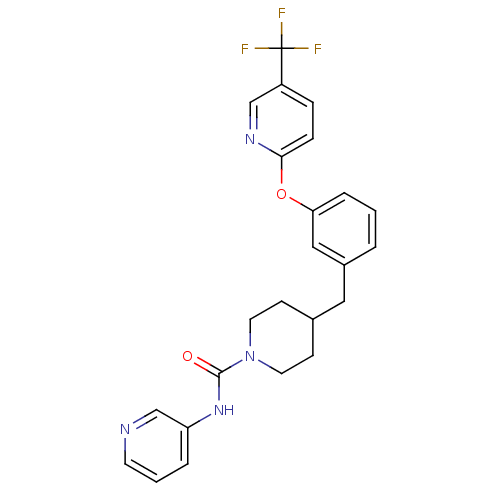 (PF-3845, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587467 (CHEMBL5094294) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587464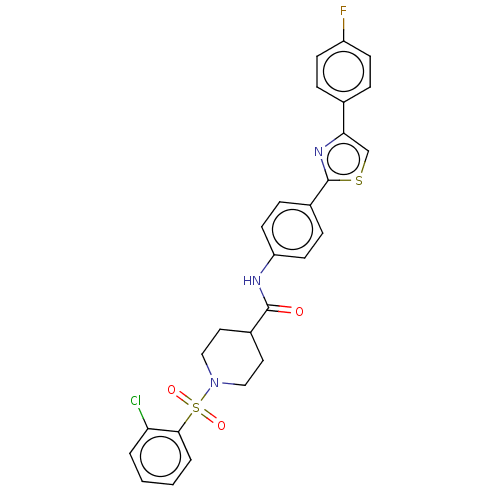 (CHEMBL5074748) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587466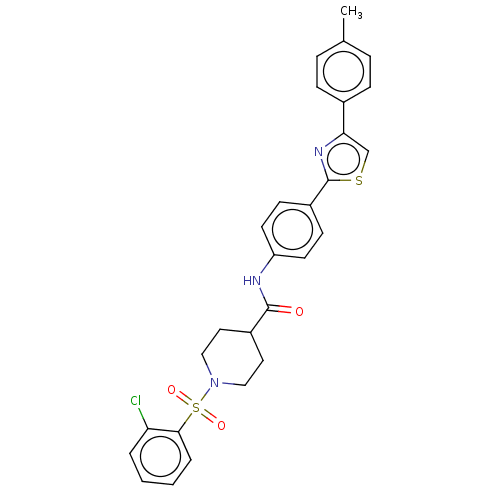 (CHEMBL5076329) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587463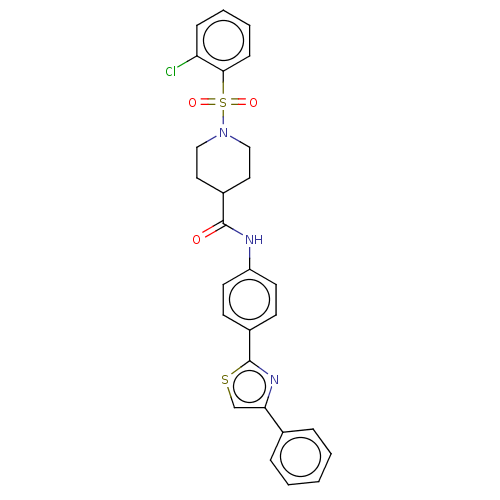 (CHEMBL5082839) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587452 (CHEMBL5089361) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587459 (CHEMBL5092295) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587457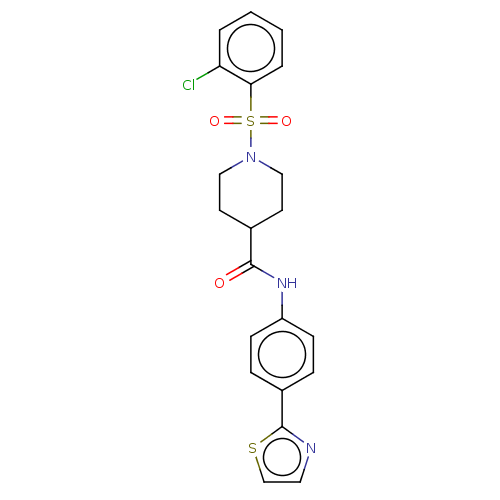 (CHEMBL5083307) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587452 (CHEMBL5089361) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587465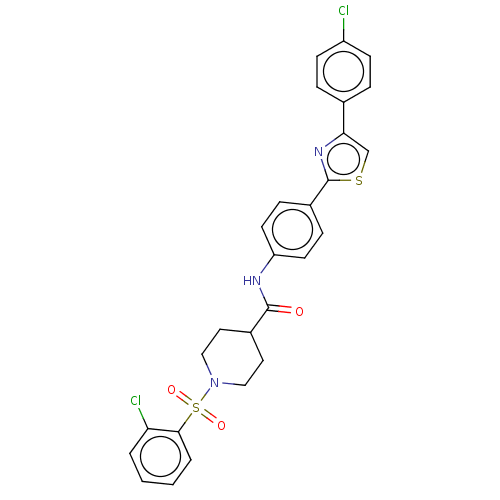 (CHEMBL5093204) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587466 (CHEMBL5076329) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587467 (CHEMBL5094294) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587464 (CHEMBL5074748) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587461 (CHEMBL5086741) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587465 (CHEMBL5093204) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587463 (CHEMBL5082839) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26739 (3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587457 (CHEMBL5083307) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587456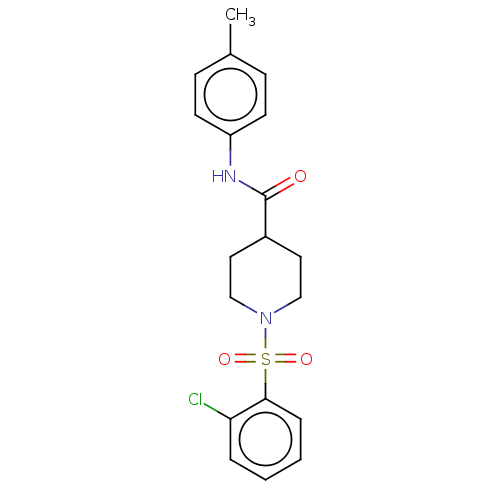 (CHEMBL5089943) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587455 (CHEMBL5075955) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587458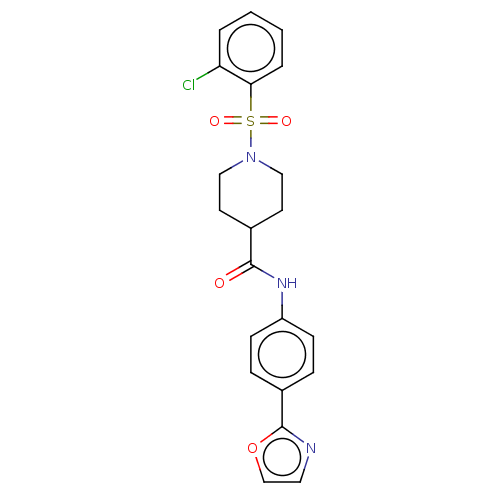 (CHEMBL5082030) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587461 (CHEMBL5086741) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587453 (CHEMBL5081733) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587462 (CHEMBL5083500) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587458 (CHEMBL5082030) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587454 (CHEMBL5088092) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587460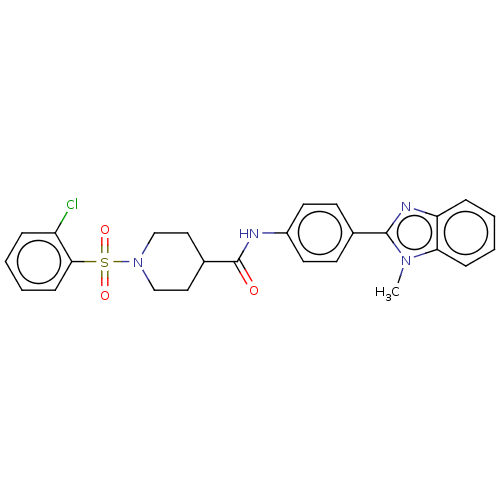 (CHEMBL5072765) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587451 (CHEMBL5076877) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587460 (CHEMBL5072765) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587456 (CHEMBL5089943) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587455 (CHEMBL5075955) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587453 (CHEMBL5081733) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587454 (CHEMBL5088092) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50587451 (CHEMBL5076877) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using cyano(6-methoxynaphthalen-2-yl)methyl((3-phenyloxiran-2-yl)methyl)carbonate as a substrate assessed as reduction in 6-m... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50587462 (CHEMBL5083500) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FAAH using N-(6-methoxypyridin-3-yl)octanamide as a substrate assessed as reduction in 6-methoxypyridin-3-amine formation by fluo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116507 BindingDB Entry DOI: 10.7270/Q2CR5Z8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||